1. We examined how different elements combine to form compounds using two models, the Periodic Table and Lewis diagrams. Looking back to the macromolecules in Lesson 2, what type(s) of compounds do you think are formed by these macromolecules? 2. What elements make up the macromolecules in Lesson 27 How could this help explain your answer to the first question?
1. We examined how different elements combine to form compounds using two models, the Periodic Table and Lewis diagrams. Looking back to the macromolecules in Lesson 2, what type(s) of compounds do you think are formed by these macromolecules? 2. What elements make up the macromolecules in Lesson 27 How could this help explain your answer to the first question?
Chapter4: Forces Between Particles
Section: Chapter Questions
Problem 4.86E
Related questions
Question
Question 1 please
Transcribed Image Text:dit was 3 minutes ago
I UA
E E X
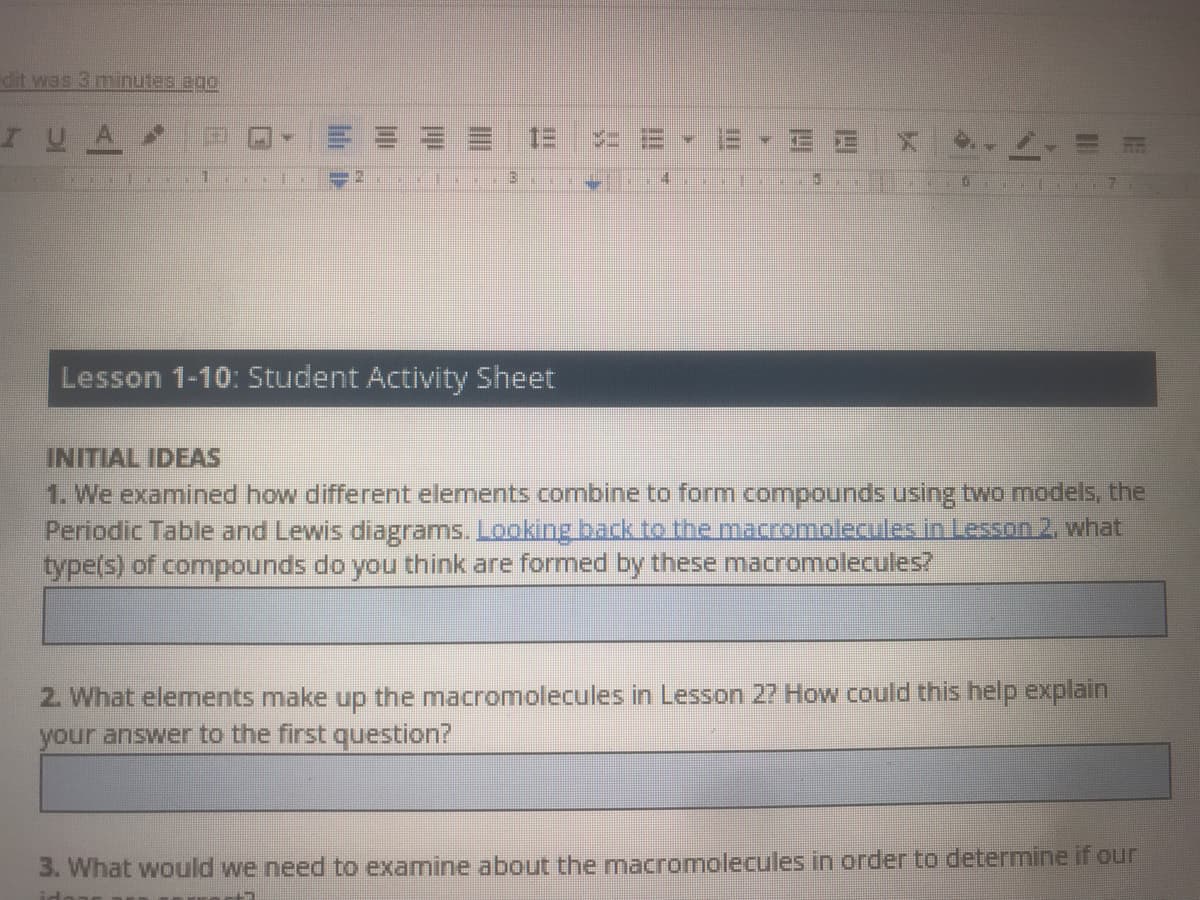
Lesson 1-10: Student Activity Sheet
INITIAL IDEAS
1. We examined how different elements combine to form compounds using two models, the
Periodic Table and Lewis diagrams. Looking back to the macromolecules in Lesson 2. what
type(s) of compounds do you think are formed by these macromolecules?
2. What elements make up the macromolecules in Lesson 27 How could this help explain
your answer to the first question?
3. What would we need to examine about the mnacromolecules in order to determine if our
lih D

Transcribed Image Text:PHOSPHOLIPIDS
All living things are made of cells. Cells all have a membrane that separates their inside and their outside.
Cell membranes are made of phospholipids lined up in a pattern. Phospholipids are surrounded by
water. The water contains charged metal ions. The phospholipids connect to each other to form a cell
membrane.
Hydrogen
Onygen
Nitrogen
Carton
Phosphorus
Hydrophtic
Head
Water
Extracelllar
Intracelklar
Hydrophobic
Tal
Phonpholipid
Image: Created by inquryHub and adapted from CC BY 4.0 mages taken trom WikIMedia Commons (Phospholpic Phosoholpid Biaver Phospholpid Struchure)
MEMBRANE PROTEINS
Membrane proteins are molecules composed of atoms of carbon, hydrogen, oxygen, nitrogen,
and sulfur. They are found within the cell membrane. Water exists on both sides of this
membrane, so the ends and middle of the proteins have to be hydrophilic while the outsides are
hydrophobic, which positions them in the middle of the membrane. Membrane proteins help
bring needed nutrients, with the help of charged metal ions, into the cell and keep it alive. About
Vs of your genome codes for these membrane proteins.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning