1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. 2. Suppose 0.140 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 18.0 °C. Calculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits. OL
1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. 2. Suppose 0.140 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 18.0 °C. Calculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits. OL
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 58QAP: The iron content of hemoglobin is determined by destroying the hemoglobin molecule and producing...
Related questions
Question
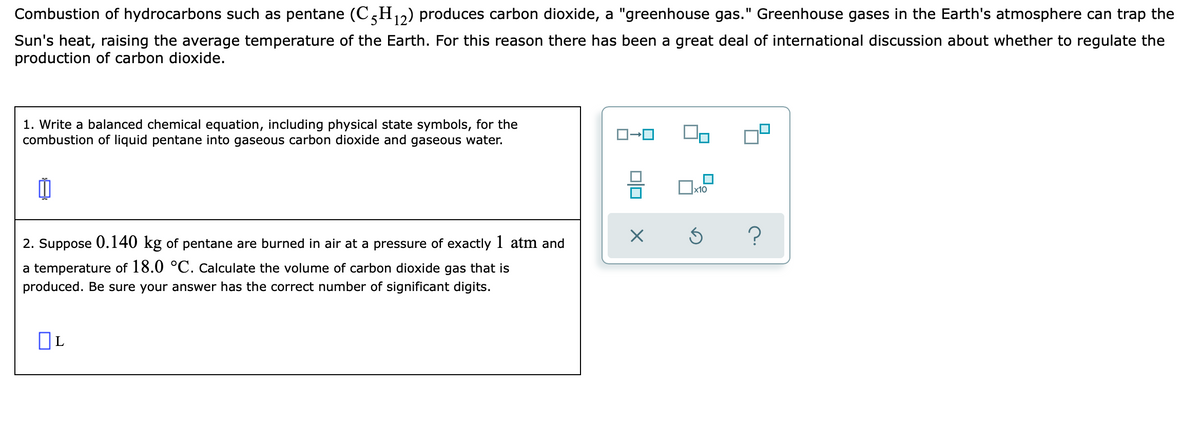
Transcribed Image Text:Combustion of hydrocarbons such as pentane (C,H,2) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the
Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the
production of carbon dioxide.
1. Write a balanced chemical equation, including physical state symbols, for the
combustion of liquid pentane into gaseous carbon dioxide and gaseous water.
2. Suppose 0.140 kg of pentane are burned in air at a pressure of exactly 1 atm and
a temperature of 18.0 °C. Calculate the volume of carbon dioxide gas that is
produced. Be sure your answer has the correct number of significant digits.
OL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning