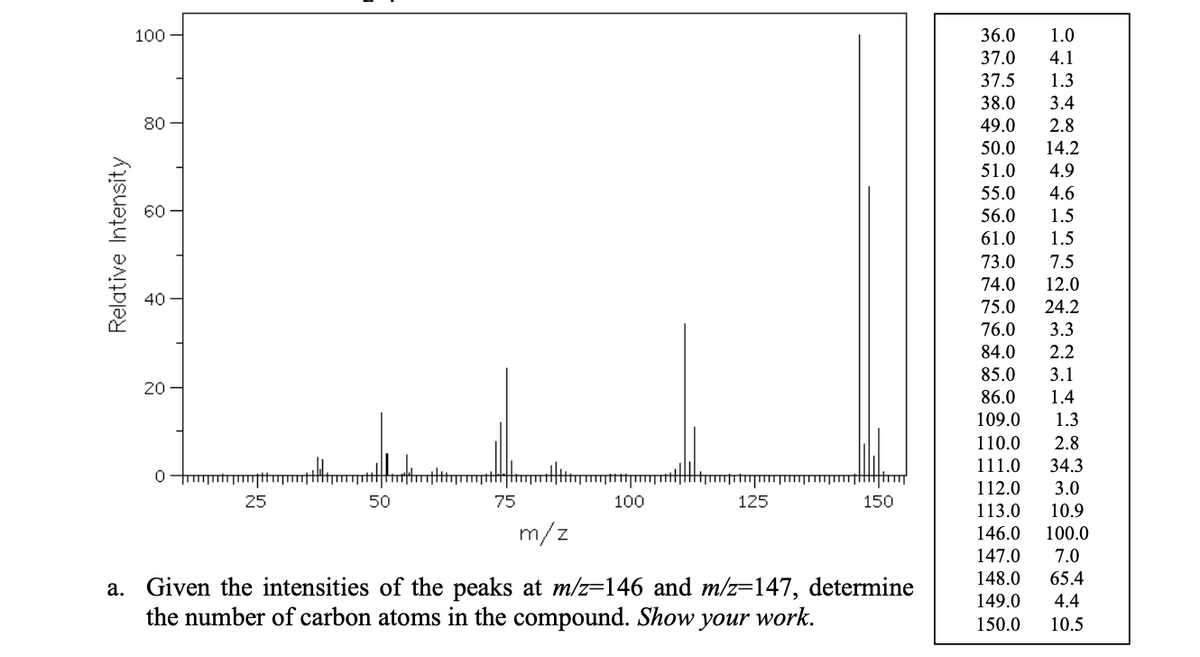
100 - 36.0 1.0 37.0 37.5 4.1 1.3 38.0 3.4 80 49.0 2.8 50.0 14.2 51.0 4.9 55.0 4.6 56.0 1.5 61.0 1.5 73.0 7.5 74.0 12.0 75.0 24.2 76.0 3.3 84.0 2.2 85.0 3.1 20 86.0 1.4 109.0 1.3 110.0 2.8 111.0 34.3 112.0 3.0 25 50 75 100 125 150 113.0 10.9 m/z 146.0 147.0 100.0 7.0 148.0 65.4 a. Given the intensities of the peaks at m/z=146 and m/z=147, determine the number of carbon atoms in the compound. Show, 149.0 4.4 уour work. 150.0 10.5 Relative Intensity
100 - 36.0 1.0 37.0 37.5 4.1 1.3 38.0 3.4 80 49.0 2.8 50.0 14.2 51.0 4.9 55.0 4.6 56.0 1.5 61.0 1.5 73.0 7.5 74.0 12.0 75.0 24.2 76.0 3.3 84.0 2.2 85.0 3.1 20 86.0 1.4 109.0 1.3 110.0 2.8 111.0 34.3 112.0 3.0 25 50 75 100 125 150 113.0 10.9 m/z 146.0 147.0 100.0 7.0 148.0 65.4 a. Given the intensities of the peaks at m/z=146 and m/z=147, determine the number of carbon atoms in the compound. Show, 149.0 4.4 уour work. 150.0 10.5 Relative Intensity
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 45E: If a sample of iron and a sample of zinc come into contact, the zinc corrodes but the iron does not....
Related questions
Question
B) Which conclusion can you draw from the presence of the cluster from m/z=146-150?
please explain and show the calculations
Transcribed Image Text:36.0
37.0
100 -
1.0
4.1
37.5
1.3
38.0
3.4
80 -
49.0
2.8
50.0
14.2
51.0
4.9
55.0
4.6
56.0
1.5
61.0
1.5
73.0
7.5
74.0
12.0
40
75.0
24.2
76.0
3.3
84.0
2.2
85.0
3.1
20-
86.0
1.4
109.0
1.3
110.0
2.8
111.0
34.3
112.0
3.0
25
50
75
100
125
150
113.0
10.9
m/z
146.0
100.0
147.0
7.0
148.0
65.4
a. Given the intensities of the peaks at m/z=146 and m/z=147, determine
the number of carbon atoms in the compound. Show
149.0
4.4
your
work.
150.0
10.5
Relative Intensity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning