100 IR Spectrum (liquid film) 4000 28 8 8 % of base peak 1738 3000 2000 1600 1200 800 V (cm³) M+* = 84 Problem 11 Mass Spectrum No significant UV absorption above 220 nm C5H8O 40 80 120 160 200 240 280 m/e 13C NMR Spectrum (100.0 MHz, CDCl 3 solution) DEPT CH2 CH3 CH↑ proton decoupled solvent 200 160 120 80 40 40 1H NMR Spectrum (400 MHz, CDCI, solution) L 10 10 9 8 7 6 100 expansion 2.2 1.8 PPM 10 5 0 8 (ppm) TMS 4 3 2 0 8 (ppm)
100 IR Spectrum (liquid film) 4000 28 8 8 % of base peak 1738 3000 2000 1600 1200 800 V (cm³) M+* = 84 Problem 11 Mass Spectrum No significant UV absorption above 220 nm C5H8O 40 80 120 160 200 240 280 m/e 13C NMR Spectrum (100.0 MHz, CDCl 3 solution) DEPT CH2 CH3 CH↑ proton decoupled solvent 200 160 120 80 40 40 1H NMR Spectrum (400 MHz, CDCI, solution) L 10 10 9 8 7 6 100 expansion 2.2 1.8 PPM 10 5 0 8 (ppm) TMS 4 3 2 0 8 (ppm)
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter20: Molecular Mass Spectrometry
Section: Chapter Questions
Problem 20.11QAP
Related questions
Question
Determine the structure of the unknown using the data provided, thank you.
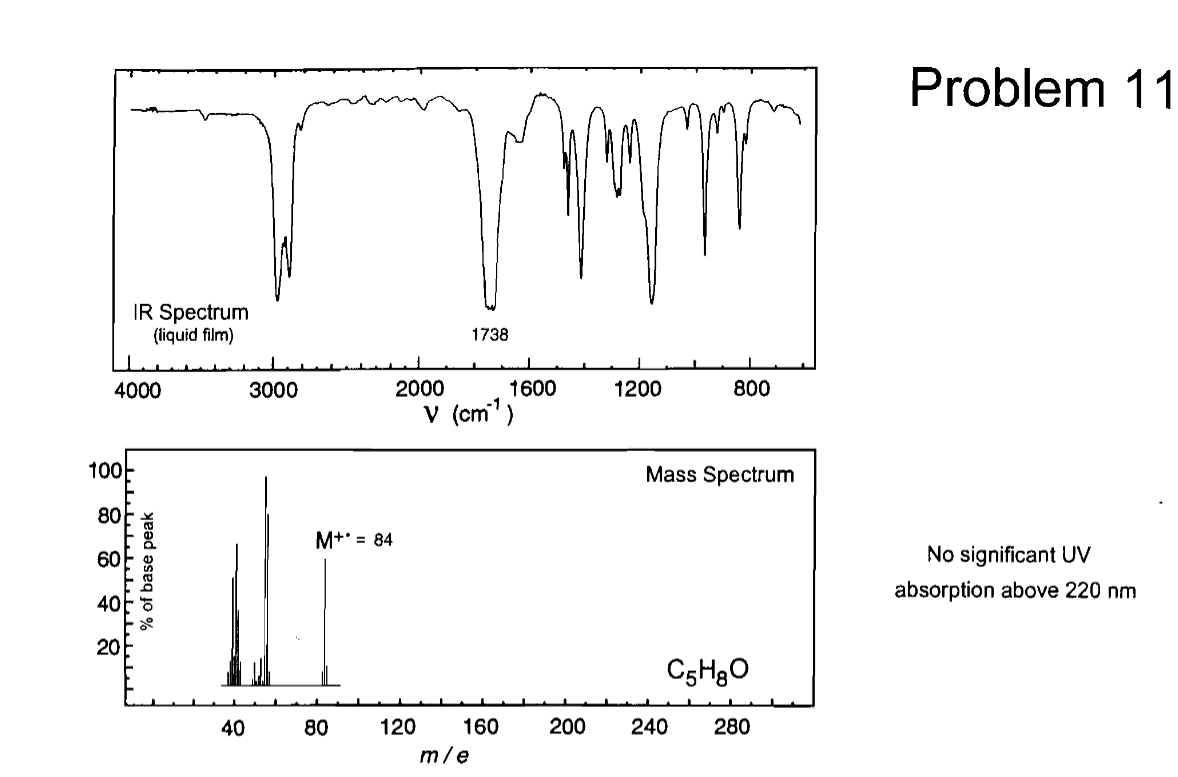
Transcribed Image Text:100
IR Spectrum
(liquid film)
4000
28 8 8
% of base peak
1738
3000
2000
1600
1200
800
V (cm³)
M+* = 84
Problem 11
Mass Spectrum
No significant UV
absorption above 220 nm
C5H8O
40
80
120
160
200
240
280
m/e
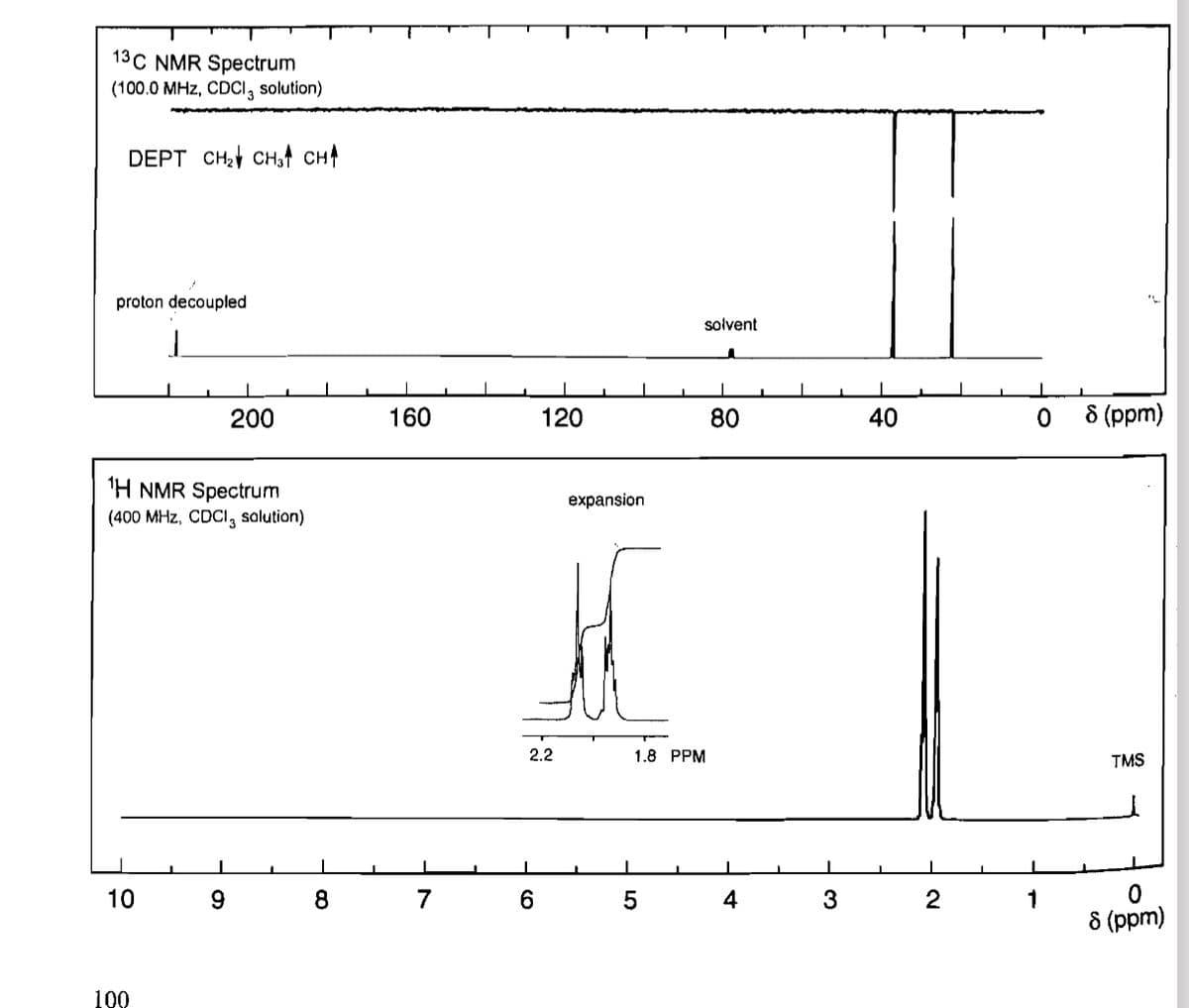
Transcribed Image Text:13C NMR Spectrum
(100.0 MHz, CDCl 3 solution)
DEPT CH2 CH3 CH↑
proton decoupled
solvent
200
160
120
80
40
40
1H NMR Spectrum
(400 MHz, CDCI, solution)
L
10
10
9
8
7
6
100
expansion
2.2
1.8 PPM
10
5
0
8 (ppm)
TMS
4
3
2
0
8 (ppm)
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning