11. The free energy of the two biomolecules is higher when they are not interacting as compared to the free energy of the two biomolecules when they come together. 12. Changing a positive charge in one of the biomolecules to a negative charge could lead to a positive change in enthalpy, which would lead to a more negative change in free energy. 13. Addition of hydrogen bonding partners on the two biomolecules could lead to a more stable interaction and thus a more negative change in free energy. 14. The interaction between the two biomolecules is likely an entropically driven process. 15. As the stability of the interaction between the two biomolecules increases the free energy change for their binding becomes more exergonic.
11. The free energy of the two biomolecules is higher when they are not interacting as compared to the free energy of the two biomolecules when they come together. 12. Changing a positive charge in one of the biomolecules to a negative charge could lead to a positive change in enthalpy, which would lead to a more negative change in free energy. 13. Addition of hydrogen bonding partners on the two biomolecules could lead to a more stable interaction and thus a more negative change in free energy. 14. The interaction between the two biomolecules is likely an entropically driven process. 15. As the stability of the interaction between the two biomolecules increases the free energy change for their binding becomes more exergonic.
Biology: The Dynamic Science (MindTap Course List)
4th Edition
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Chapter6: Energy, Enzymes, And Biological Reactions
Section: Chapter Questions
Problem 6TYK: Which of the following methods is not used by enzymes to increase the rate of reactions? a. covalent...
Related questions
Question
Could you tell me whether these statements are true or false and why?
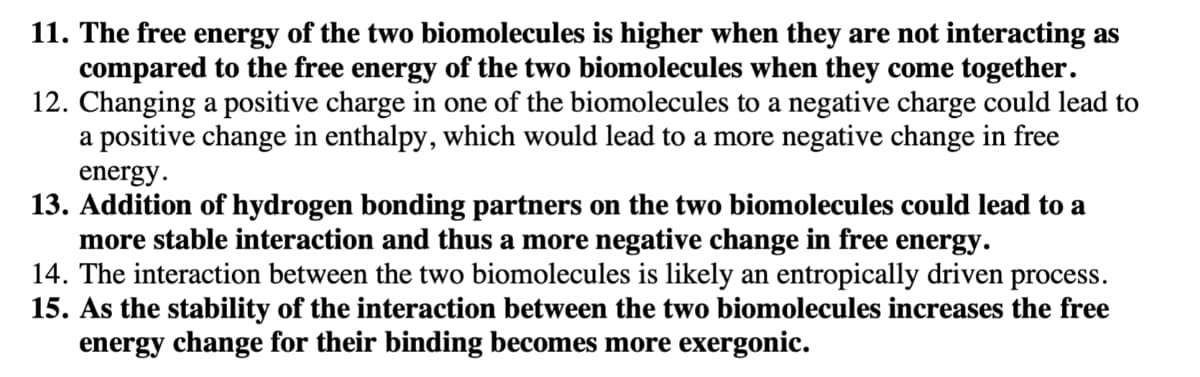
Transcribed Image Text:11. The free energy of the two biomolecules is higher when they are not interacting as
compared to the free energy of the two biomolecules when they come together.
12. Changing a positive charge in one of the biomolecules to a negative charge could lead to
a positive change in enthalpy, which would lead to a more negative change in free
energy.
13. Addition of hydrogen bonding partners on the two biomolecules could lead to a
more stable interaction and thus a more negative change in free energy.
14. The interaction between the two biomolecules is likely an entropically driven process.
15. As the stability of the interaction between the two biomolecules increases the free
energy change for their binding becomes more exergonic.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Nutrition: Concepts and Controversies - Standalo…
Health & Nutrition
ISBN:
9781305627994
Author:
Frances Sizer, Ellie Whitney
Publisher:
Brooks Cole

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Nutrition: Concepts and Controversies - Standalo…
Health & Nutrition
ISBN:
9781305627994
Author:
Frances Sizer, Ellie Whitney
Publisher:
Brooks Cole

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781337408332
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781305073951
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning