11.) Which of the following entropies are arrange in an increasing order? A. gas < solid < liquid C. liquid < solid < gas B. solid < liquid < gas D. gas < liquid < solid 12) Whieh
11.) Which of the following entropies are arrange in an increasing order? A. gas < solid < liquid C. liquid < solid < gas B. solid < liquid < gas D. gas < liquid < solid 12) Whieh
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter22: Surfaces
Section: Chapter Questions
Problem 22.10E
Related questions
Question
Please answer 11-14.
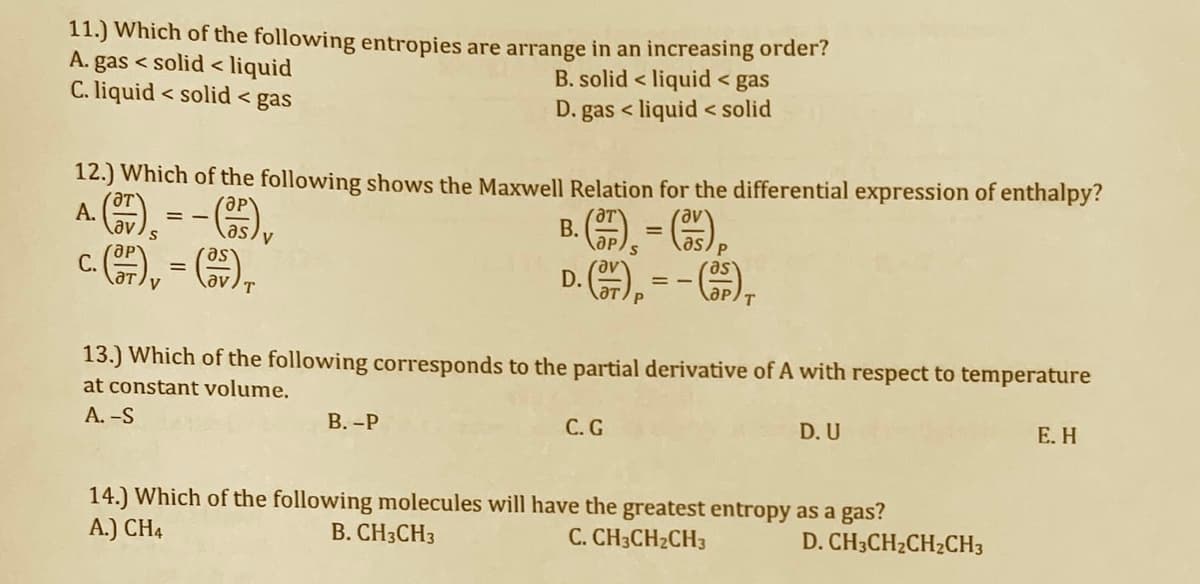
Transcribed Image Text:11.) Which of the following entropies are arrange in an increasing order?
A. gas < solid < liquid
C. liquid < solid < gas
B. solid < liquid < gas
D. gas < liquid < solid
12.) Which of the following shows the Maxwell Relation for the differential expression of enthalpy?
A ), --),
c.A, - G),
А.
В.
as
as
P
as
С.
D.
P
13.) Which of the following corresponds to the partial derivative of A with respect to temperature
at constant volume.
А. -S
В.-Р
С.G
D. U
Е. Н
14.) Which of the following molecules will have the greatest entropy as a gas?
A.) CH4
B. CH3CH3
C. CH3CH2CH3
D. CH3CH2CH2CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning