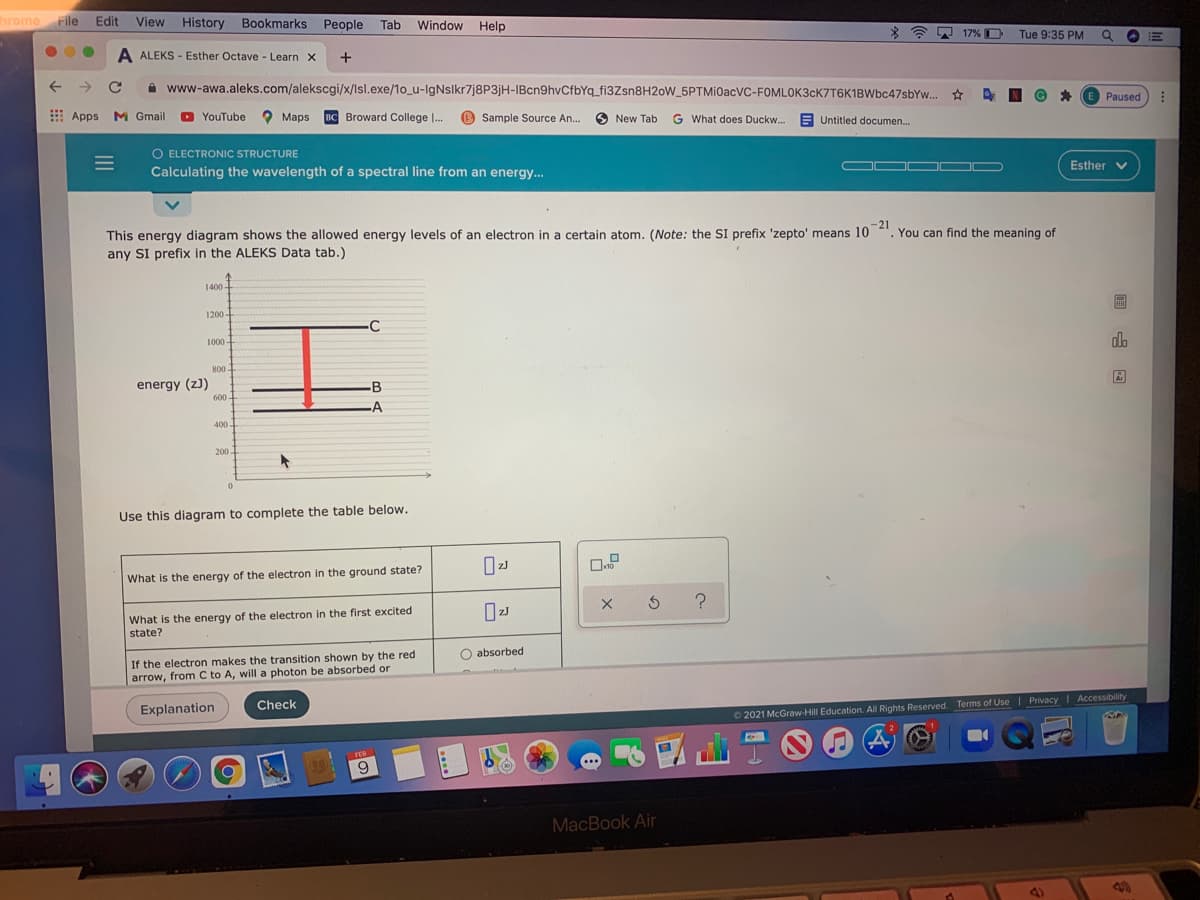
This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10 ". You can find the meaning of any SI prefix in the ALEKS Data tab.) 1400 1200 - -C 1000- 800 energy (z)) 600 - -B A 400- 200. Use this diagram to complete the table below. What is the energy of the electron in the ground state? What is the energy of the electron in the first excited state? If the electron makes the transition shown by the red arrow, from C to A, will a photon be absorbed or O absorbed
This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10 ". You can find the meaning of any SI prefix in the ALEKS Data tab.) 1400 1200 - -C 1000- 800 energy (z)) 600 - -B A 400- 200. Use this diagram to complete the table below. What is the energy of the electron in the ground state? What is the energy of the electron in the first excited state? If the electron makes the transition shown by the red arrow, from C to A, will a photon be absorbed or O absorbed
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter5: Gases
Section: Chapter Questions
Problem 129E
Related questions
Question
Transcribed Image Text:hrome
File
Edit
View History Bookmarks People
Tab
Window Help
17% O
Tue 9:35 PM
A ALEKS - Esther Octave Learn X
+
A www-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IBcn9hvCfbYq_fi3Zsn8H2oW_5PTMI0acVC-FOMLOK3cK7T6K1BWbc47sbYw. *
Paused
E Apps M Gmail
YouTube Maps
BC Broward College |.
Sample Source An...
O New Tab
G What does Duckw.. E Untitled documen...
O ELECTRONIC STRUCTURE
OO
Esther
Calculating the wavelength of a spectral line from an energy..
-21
". You can find the meaning of
This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10
any SI prefix in the ALEKS Data tab.)
1400 -
1200 -
-C
dlo
1000 -
800
Ar
energy (z)
B
600 -
-A
400.
200-
Use this diagram to complete the table below.
What is the energy of the electron in the ground state?
What is the energy of the electron in the first excited
state?
O absorbed
If the electron makes the transition shown by the red
arrow, from C to A, will a photon be absorbed or
Check
Explanation
O2021 McGraw-Hill Education. All Rights Reserved. Terms of Use PrivacyI Accessibility
FEB
9
MacBook Air
4)
III
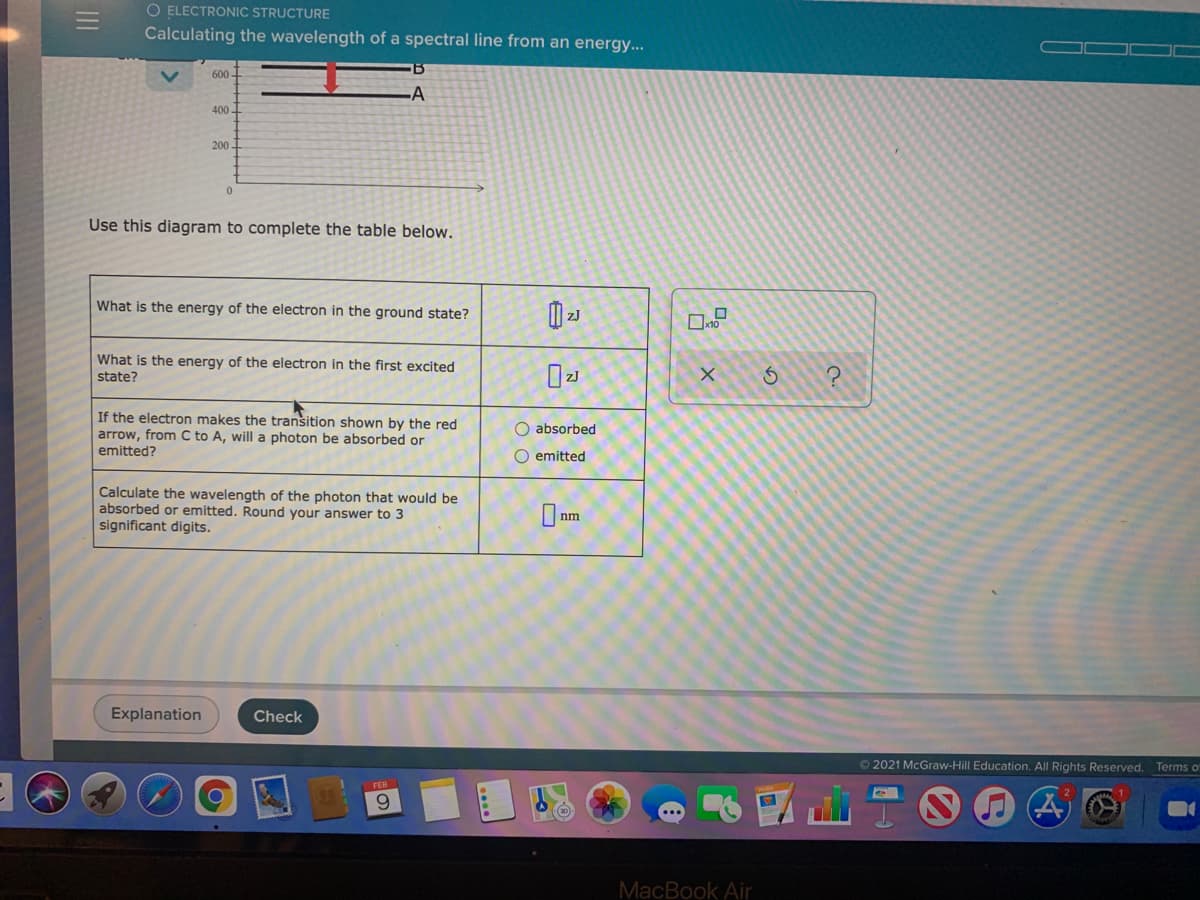
Transcribed Image Text:O ELECTRONIC STRUCTURE
Calculating the wavelength of a spectral line from an energy...
600 -
-A
400-
200-
Use this diagram to complete the table below.
What is the energy of the electron in the ground state?
What is the energy of the electron in the first excited
state?
If the electron makes the tranšition shown by the red
arrow, from C to A, will a photon be absorbed or
emitted?
O absorbed
O emitted
Calculate the wavelength of the photon that would be
absorbed or emitted. Round your answer to 3
significant digits.
Explanation
Check
© 2021 McGraw-Hill Education. All Rights Reserved.
Terms o
山
9.
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning