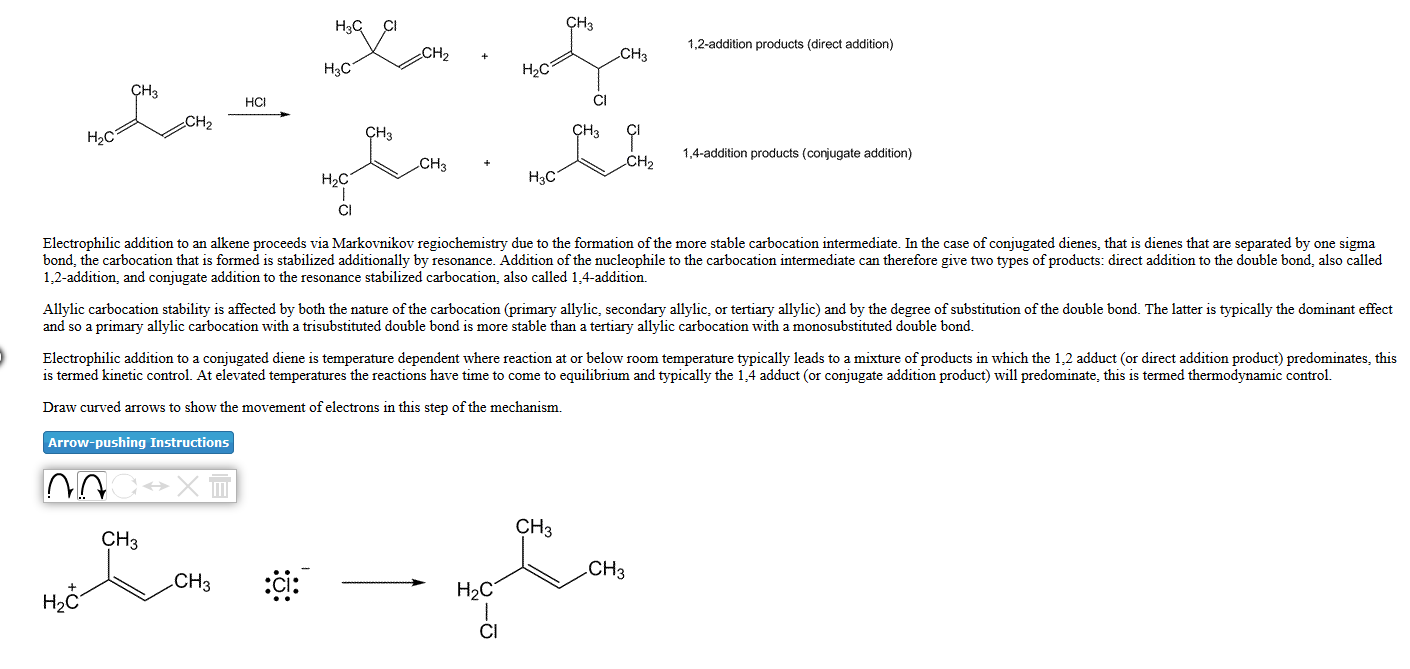
1,2-addition products (direct addition) CH H3C H2C HCI H2C 1,4-addition products (conjugate addition) CH3 H2C H3C Electrophilic addition to an alkene proceeds via Markovnikov regiochemistry due to the formation of the more stable carbocation intermediate. In the case of conjugated dienes, that is dienes that are separated by one sigma bond, the carbocation that is formed is stabilized additionally by resonance. Addition of the nucleophile to the carbocation intermediate can therefore give two types of products: direct addition to the double bond, also called 1,2-addition, and conjugate addition to the resonance stabilized carbocation, also called 1,4-addition. Allylic carbocation stability is affected by both the nature of the carbocation (primary allylic, secondary allylic, or tertiary allylic) and by the degree of substitution of the double bond. The latter is typically the dominant effect and so a primary allylic carbocation with a trisubstituted double bond is more stable than a tertiary allylic carbocation with a monosubstituted double bond. Electrophilic addition to a conjugated diene is temperature dependent where reaction at or below room temperature typically leads to a mixture of products in which the 1,2 adduct (or direct addition product) predominates, this is termed kinetic control. At elevated temperatures the reactions have time to come to equilibrium and typically the 1,4 adduct (or conjugate addition product) will predominate, this is termed thermodynamic control. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions CHa CH CH3 CH H2C H2C
Reactions of Ethers
Ethers (R-O-R’) are compounds formed by replacing hydrogen atoms of an alcohol (R-OH compound) or a phenol (C6H5OH) by an aryl/ acyl group (functional group after removing single hydrogen from an aromatic ring). In this section, reaction, preparation and behavior of ethers are discussed in the context of organic chemistry.
Epoxides
Epoxides are a special class of cyclic ethers which are an important functional group in organic chemistry and generate reactive centers due to their unusual high reactivity. Due to their high reactivity, epoxides are considered to be toxic and mutagenic.
Williamson Ether Synthesis
An organic reaction in which an organohalide and a deprotonated alcohol forms ether is known as Williamson ether synthesis. Alexander Williamson developed the Williamson ether synthesis in 1850. The formation of ether in this synthesis is an SN2 reaction.
Electrophilic addition to an
Allylic carbocation stability is affected by both the nature of the carbocation (primary allylic, secondary allylic, or tertiary allylic) and by the degree of substitution of the double bond. The latter is typically the dominant effect and so a primary allylic carbocation with a trisubstituted double bond is more stable than a tertiary allylic carbocation with a monosubstituted double bond.
Electrophilic addition to a conjugated diene is temperature dependent where reaction at or below room temperature typically leads to a mixture of products in which the 1,2 adduct (or direct addition product) predominates, this is termed kinetic control. At elevated temperatures the reactions have time to come to equilibrium and typically the 1,4 adduct (or conjugate addition product) will predominate, this is termed
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images









