140 120 Super- critical fluid A 100 CO,(s) Co,(1) 80 Critical point 30.98°C, 60 72.79 atm 40 B 20 CO,(g) Triple point -56.57°C, 5.11 atm -80 -60 -40 -20 40 Temperature (C) What phase of carbon dioxide has the lowest chemical potential at 100 bar and 320 K? O cole) supercritical CO, O all CO, phases have the same chemical potential O co2) Pressure (atm) 20
140 120 Super- critical fluid A 100 CO,(s) Co,(1) 80 Critical point 30.98°C, 60 72.79 atm 40 B 20 CO,(g) Triple point -56.57°C, 5.11 atm -80 -60 -40 -20 40 Temperature (C) What phase of carbon dioxide has the lowest chemical potential at 100 bar and 320 K? O cole) supercritical CO, O all CO, phases have the same chemical potential O co2) Pressure (atm) 20
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 128IP: Some nonelectrolyte solute (molar mass = 142 g/mol) was dissolved in 150. mL of a solvent (density =...
Related questions
Question
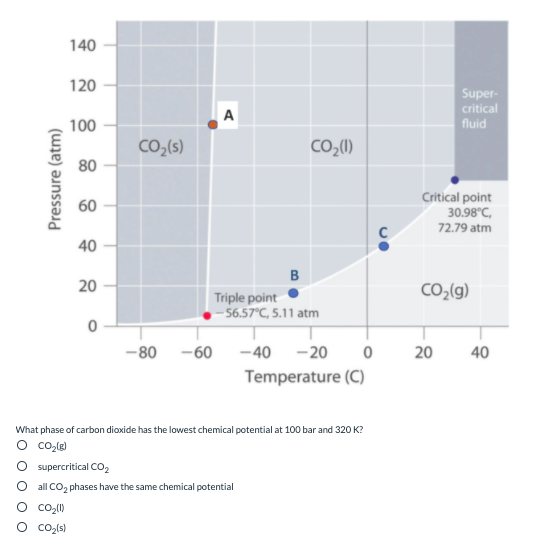
Transcribed Image Text:140
120
Super-
critical
fluid
A
100
CO,(s)
CO,(1)
80
Critical point
30.98°C,
60
72.79 atm
40
B
20
CO2(g)
Triple point
– 56.57°C, 5.11 atm
-80 -60
-40 -20
20
40
Temperature (C)
What phase of carbon dioxide has the lowest chemical potential at 100 bar and 320 K?
O cole)
supercritical CO,
O all CO, phases have the same chemical potential
O co2/s)
Pressure (atm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,