2) dimethylcyclopentane. To accomplish their syntheses, Poly chooses to start from a trisubstituted alkene while B-Ryan chooses to start from a disubstituted alkene isomer. Poly's synthesis provides a mixture of isomers while B-Ryan's provides two stereoisomers. Both synthetic approaches are shown below. Poly and B-Ryan are asked by their supervisor to synthesize (1R,3R)-1-chloro-1,3- Me Me. Ме .CI A Me HCI „Me B-Ryan's Synthesis 'CI + 75% 25% Me, Me. Me., Me, HCI .CI Me B Me Poly's Synthesis -Me "Me 'CI + + Me., a trace mixture: jof constitutional: chloroalkane isomers .CI Me Using your mechanistic knowledge of alkene reactions: (a) Explain the selectivity (regio and stereo) observed in B-Ryan's synthesis. (b) Propose a mechanism that accounts the formation of isomer C in Poly's synthesis. (c) Draw the major stereoisomer(s) formed in the "trace mixture of constitutional isomers" isolated in Poly's synthesis.
2) dimethylcyclopentane. To accomplish their syntheses, Poly chooses to start from a trisubstituted alkene while B-Ryan chooses to start from a disubstituted alkene isomer. Poly's synthesis provides a mixture of isomers while B-Ryan's provides two stereoisomers. Both synthetic approaches are shown below. Poly and B-Ryan are asked by their supervisor to synthesize (1R,3R)-1-chloro-1,3- Me Me. Ме .CI A Me HCI „Me B-Ryan's Synthesis 'CI + 75% 25% Me, Me. Me., Me, HCI .CI Me B Me Poly's Synthesis -Me "Me 'CI + + Me., a trace mixture: jof constitutional: chloroalkane isomers .CI Me Using your mechanistic knowledge of alkene reactions: (a) Explain the selectivity (regio and stereo) observed in B-Ryan's synthesis. (b) Propose a mechanism that accounts the formation of isomer C in Poly's synthesis. (c) Draw the major stereoisomer(s) formed in the "trace mixture of constitutional isomers" isolated in Poly's synthesis.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter13: Substitution
Section: Chapter Questions
Problem 4E
Related questions
Question
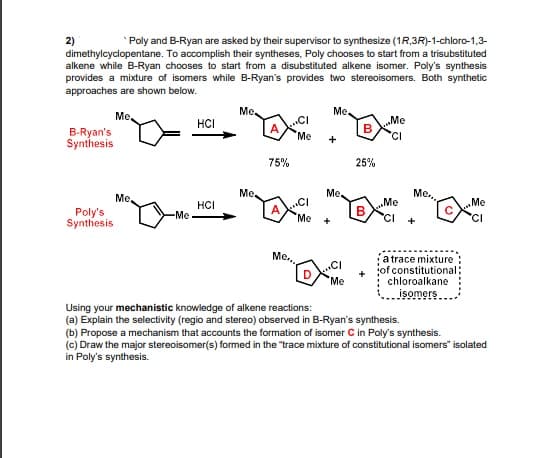
Transcribed Image Text:2)
dimethylcyclopentane. To accomplish their syntheses, Poly chooses to start from a trisubstituted
alkene while B-Ryan chooses to start from a disubstituted alkene isomer. Poly's synthesis
provides a mixture of isomers while B-Ryan's provides two stereoisomers. Both synthetic
approaches are shown below.
Poly and B-Ryan are asked by their supervisor to synthesize (1R,3R)-1-chloro-1,3-
Me
Ме.
Me,
.CI
A
Me
HCI
„Me
B-Ryan's
Synthesis
'CI
+
75%
25%
Me,
Me.
Me.,
Me,
.CI
'Me
Me
HCI
-Me
B Me
Poly's
Synthesis
'CI
+
+
Me.,
.CI
Me
a trace mixture :
jof constitutional:
chloroalkane
isomers
Using your mechanistic knowledge of alkene reactions:
(a) Explain the selectivity (regio and stereo) observed in B-Ryan's synthesis.
(b) Propose a mechanism that accounts the formation of isomer C in Poly's synthesis.
(c) Draw the major stereoisomer(s) formed in the "trace mixture of constitutional isomers" isolated
in Poly's synthesis.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning