2) i) Draw a perspective diagram (dash/wedge) of (S)-1-bromo-1-fluoroethane. Only use dash/wedge for the chirality center, and show your C.I.P. priorities to prove the (S)- designation. i) Draw a detailed mechanism showing the formation of the major product(s) of the reaction between (S)-1-bromo-1-fluoroethane and sodium methoxide (NAOCH, the Na" is a spectator). Please be sure to include all structures (use perspective diagrams as appropriate to illustrate the stereochemistry of the process), resonance forms, intermediates, transition states, curved arrows, formal charges, or lone pairs as necessary. iii) Please redraw your product(s) below and assign (R/S) to the chirality center(s) therein. (please show your C.I.P priorities). Is the mechanism SN1 or SN2, and what is the overall stereochemistry associated with the process? Why is this an interesting case?
2) i) Draw a perspective diagram (dash/wedge) of (S)-1-bromo-1-fluoroethane. Only use dash/wedge for the chirality center, and show your C.I.P. priorities to prove the (S)- designation. i) Draw a detailed mechanism showing the formation of the major product(s) of the reaction between (S)-1-bromo-1-fluoroethane and sodium methoxide (NAOCH, the Na" is a spectator). Please be sure to include all structures (use perspective diagrams as appropriate to illustrate the stereochemistry of the process), resonance forms, intermediates, transition states, curved arrows, formal charges, or lone pairs as necessary. iii) Please redraw your product(s) below and assign (R/S) to the chirality center(s) therein. (please show your C.I.P priorities). Is the mechanism SN1 or SN2, and what is the overall stereochemistry associated with the process? Why is this an interesting case?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter1: Introduction
Section: Chapter Questions
Problem 1.12QAP
Related questions
Question
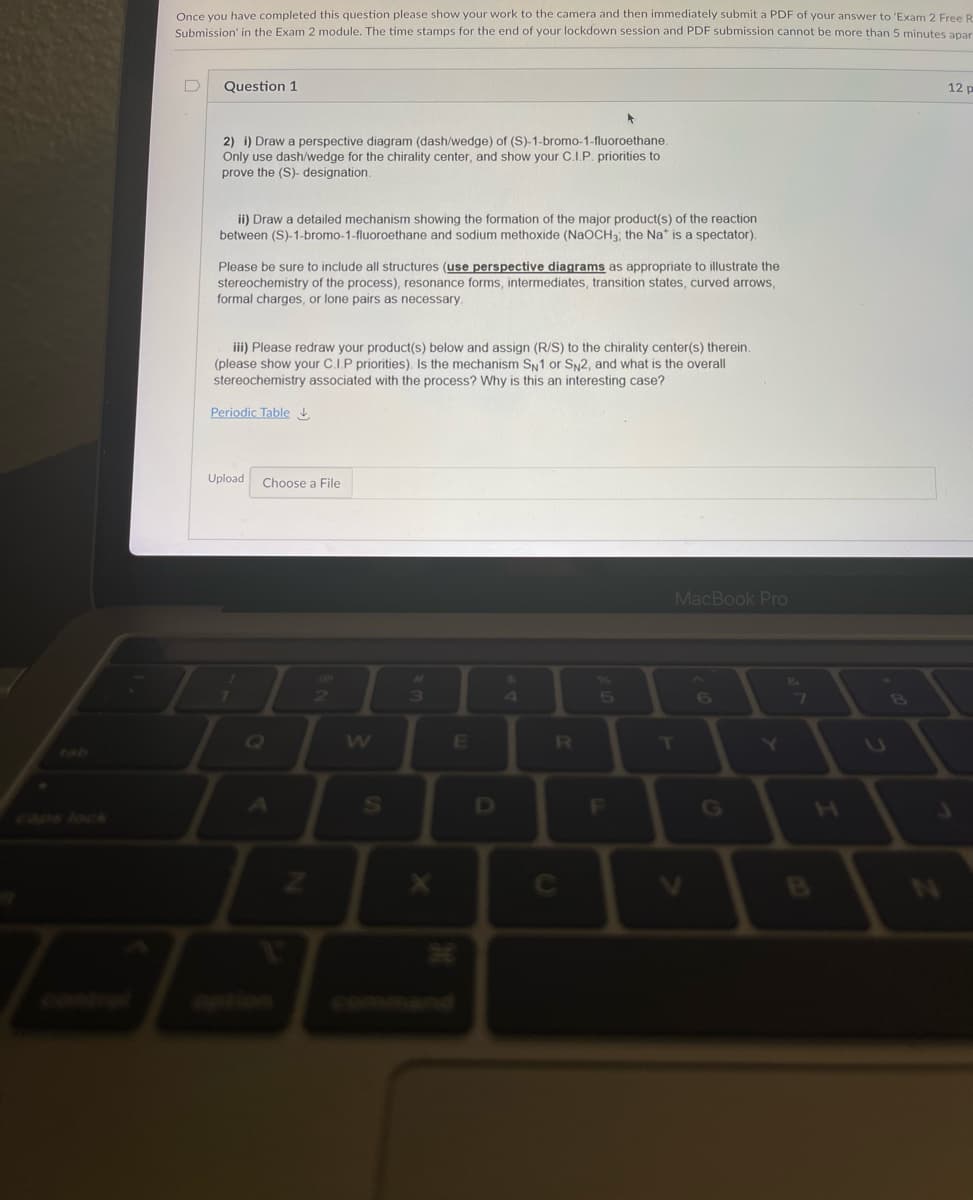
Transcribed Image Text:Once you have completed this question please show your work to the camera and then immediately submit a PDF of your answer to 'Exam 2 Free R
Submission' in the Exam 2 module. The time stamps for the end of your lockdown session and PDF submission cannot be more than 5 minutes apar
D
Question 1
12 p
2) i) Draw a perspective diagram (dash/wedge) of (S)-1-bromo-1-fluoroethane.
Only use dash/wedge for the chirality center, and show your C.I.P. priorities to
prove the (S)- designation.
ii) Draw a detailed mechanism showing the formation of the major product(s) of the reaction
between (S)-1-bromo-1-fluoroethane and sodium methoxide (NaOCH3; the Na* is a spectator).
Please be sure to include all structures (use perspective diagrams as appropriate to illustrate the
stereochemistry of the process), resonance forms, intermediates, transition states, curved arrows,
formal charges, or lone pairs as necessary.
iii) Please redraw your product(s) below and assign (R/S) to the chirality center(s) therein.
(please show your C.I.P priorities). Is the mechanism Sy1 or Sy2, and what is the overall
stereochemistry associated with the process? Why is this an interesting case?
Periodic Table
Upload
Choose a File
MacBook Pro
5
6
We
E
R
tab
F
G
CADS look
C
aption
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning