2) Na,CO3(aq) + CaCl2(aq) → 2 NaCl(aq) + CaCO3(s) Calculate the volume (in mL) of 0.200 M CaCl, needed to produce 2.00 g of CaCO,(s) . There is an excess of Na2CO3. Molar mass of calcium carbonate = 100.09 g/mol Volume of calcium chloride = mL
2) Na,CO3(aq) + CaCl2(aq) → 2 NaCl(aq) + CaCO3(s) Calculate the volume (in mL) of 0.200 M CaCl, needed to produce 2.00 g of CaCO,(s) . There is an excess of Na2CO3. Molar mass of calcium carbonate = 100.09 g/mol Volume of calcium chloride = mL
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 31E
Related questions
Question
Everytime I get 100 mL but the site is saying its still not right, please help.
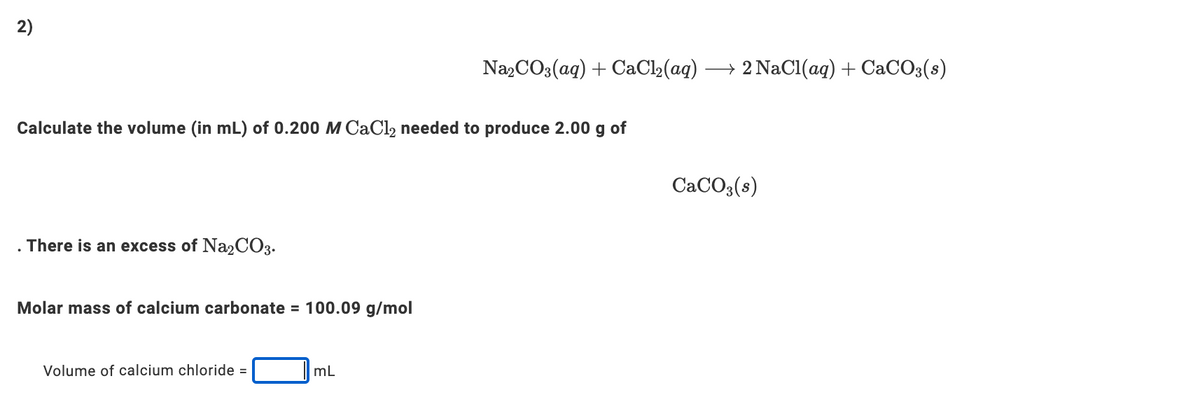
Transcribed Image Text:2)
Na,CO3(ag) + CaCl2(aq)
→ 2 NaCl(ag) + CaCO3(s)
Calculate the volume (in mL) of 0.200 M CaCl2 needed to produce 2.00 g of
CACO3(s)
There is an excess of Na2CO3.
Molar mass of calcium carbonate = 100.09 g/mol
Volume of calcium chloride =
mL
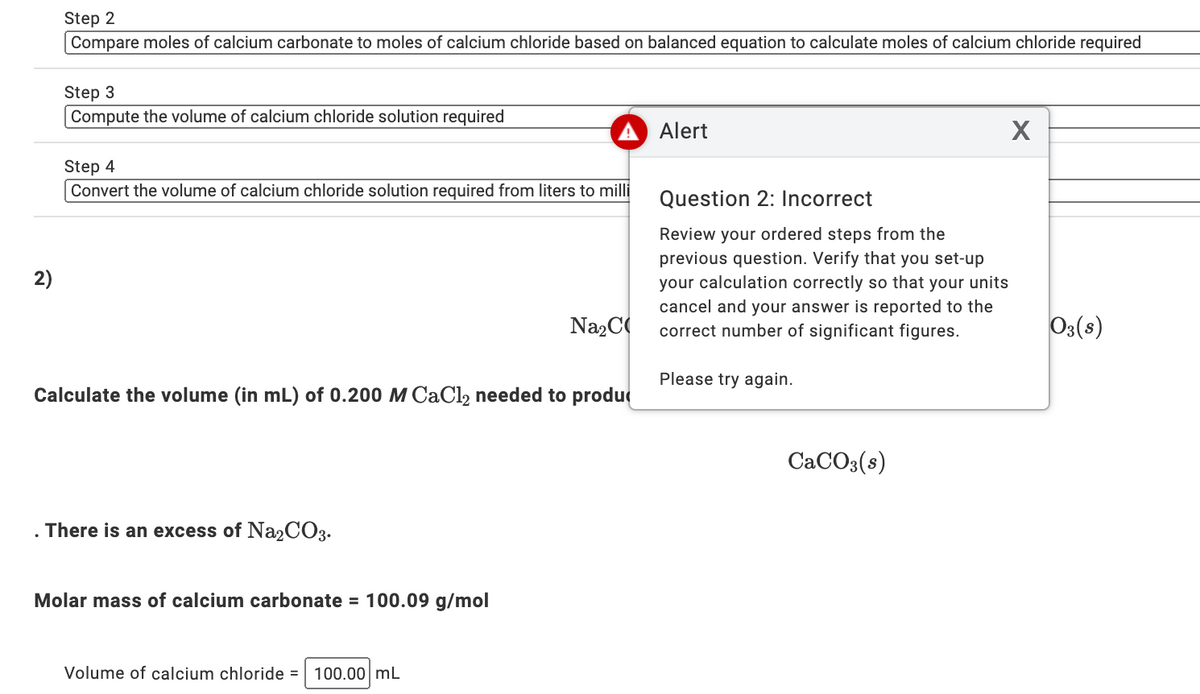
Transcribed Image Text:Step 2
Compare moles of calcium carbonate to moles of calcium chloride based on balanced equation to calculate moles of calcium chloride required
Step 3
Compute the volume of calcium chloride solution required
Alert
Step 4
Convert the volume of calcium chloride solution required from liters to milli
Question 2: Incorrect
Review your ordered steps from the
previous question. Verify that you set-up
your calculation correctly so that your units
cancel and your answer is reported to the
correct number of significant figures.
2)
Na2C
Os(s)
Please try again.
Calculate the volume (in mL) of 0.200 M CAC12 needed to produ
CACO3(s)
There is an excess of Na2CO3.
Molar mass of calcium carbonate = 100.09 g/mol
Volume of calcium chloride = 100.00 mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning