2. Balance the following combustion reaction (reaction takes place at a temperature > 100 °C) and answer the questions below. Please include the phases for CO2 and H2O in (). C4H10 (g) + CO2 ) + H20 ( Which elements can be balanced first? Which element is balanced last?
2. Balance the following combustion reaction (reaction takes place at a temperature > 100 °C) and answer the questions below. Please include the phases for CO2 and H2O in (). C4H10 (g) + CO2 ) + H20 ( Which elements can be balanced first? Which element is balanced last?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 10CR: Consider the unbalanced equation for the combustion of propane: :math>C3H8(g)+O2(g)CO2(g)+H2O(g)...
Related questions
Question
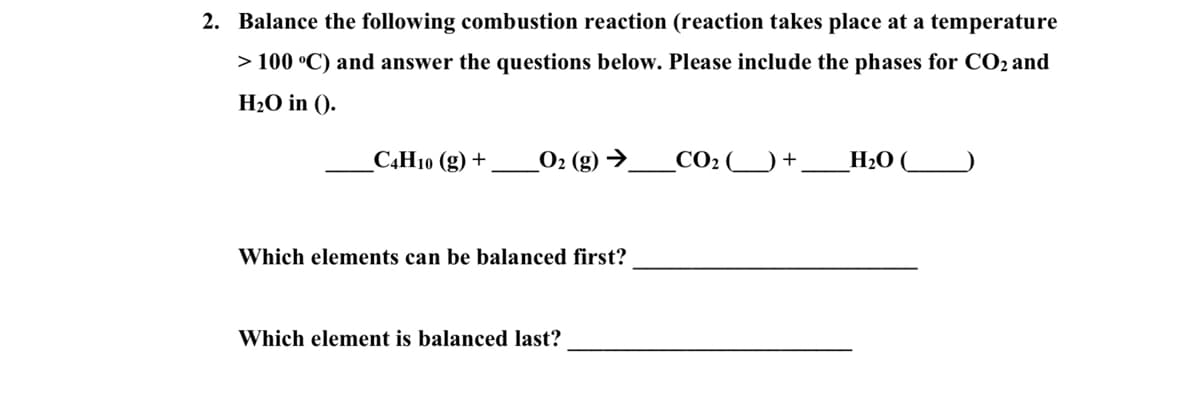
Transcribed Image Text:2. Balance the following combustion reaction (reaction takes place at a temperature
> 100 °C) and answer the questions below. Please include the phases for CO2 and
H2O in ().
САН1ю (g) +
_O2 (g) →.
CO2
H2O (
Which elements can be balanced first?
Which element is balanced last?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning