2. Consider an electron in a hydrogen atom that is transitioning from the n-6 to the n1 level. (a) Fill in the blank: In order for this transition to occur will the photon be Submit Answer Tries 0/99 (b) What amount of energy (in 3) does the photon associated with t his transition have? Submit Answer Tries 0/99 (c) What is the frequency (in Hz) of the photon associated with this transition? Hz
2. Consider an electron in a hydrogen atom that is transitioning from the n-6 to the n1 level. (a) Fill in the blank: In order for this transition to occur will the photon be Submit Answer Tries 0/99 (b) What amount of energy (in 3) does the photon associated with t his transition have? Submit Answer Tries 0/99 (c) What is the frequency (in Hz) of the photon associated with this transition? Hz
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 53AP: Photons are emitted in the Lyman series as hydrogen atoms undergo transitions from various excited...
Related questions
Question
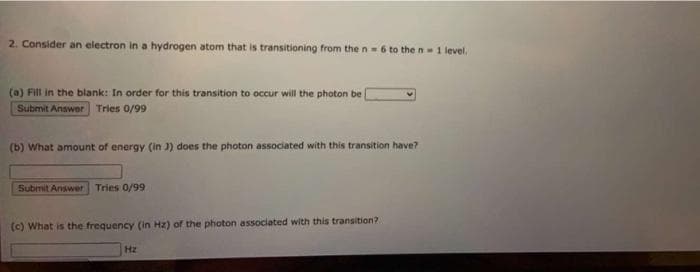
Transcribed Image Text:2. Consider an electron in a hydrogen atom that is transitioning from the n- 6 to the n 1 level.
(a) Fill in the blank: In order for this transition to occur will the photon be
Submit Answer Tries 0/99
(b) What amount of energy (in 3) does the photon associated with this transition have?
Submit Answer Tries 0/99
(c) What is the frequency (in Hz) of the photon associated with this transition?
Hz
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning