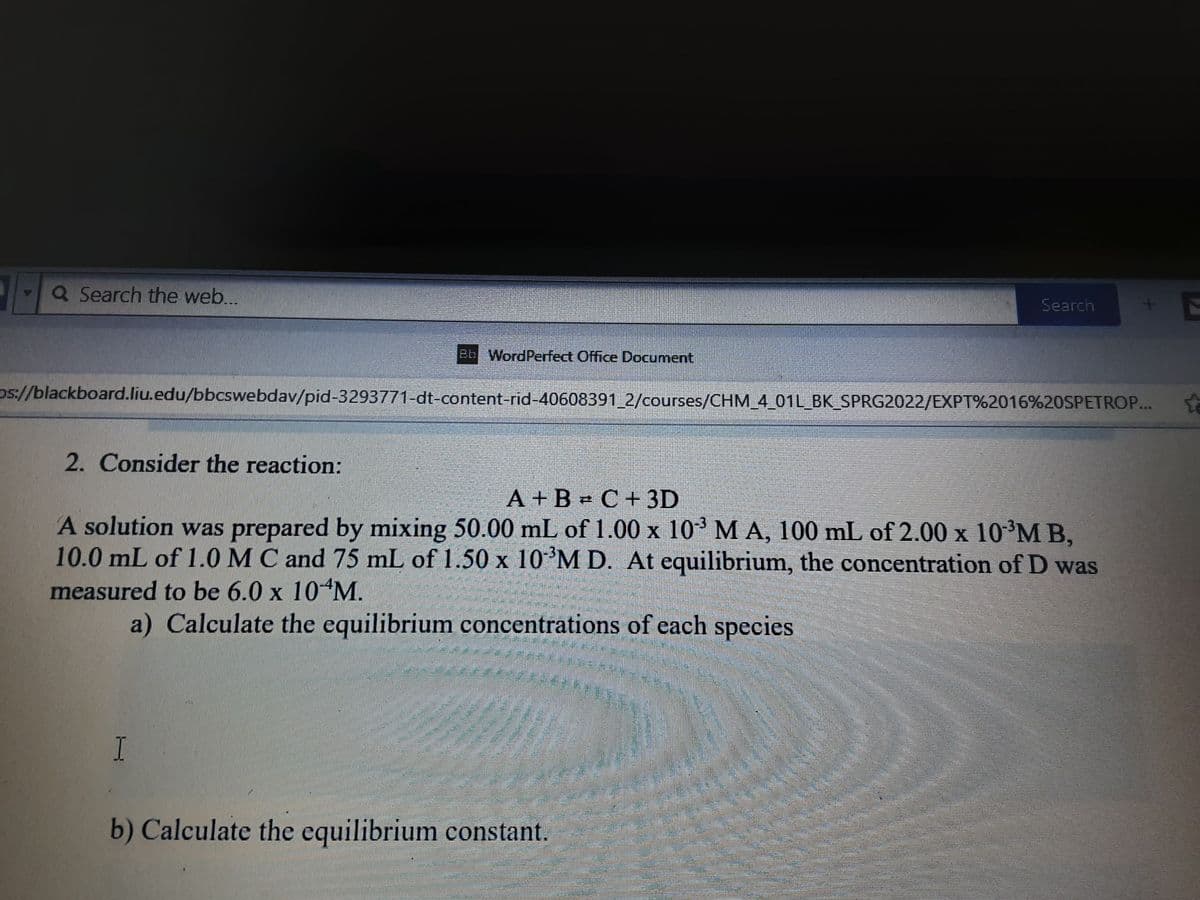
2. Consider the reaction: A + B C+ 3D A solution was prepared by mixing 50.00 mL of 1.00 x 10 M A, 100 mL of 2.00 x 10 M B, 10.0 mL of 1.0 MC and 75 mL of 1.50 x 10 M D. At equilibrium, the concentration of D was measured to be 6.0 x 10 M. a) Calculate the equilibrium concentrations of each species I b) Calculate the equilibrium constant.
2. Consider the reaction: A + B C+ 3D A solution was prepared by mixing 50.00 mL of 1.00 x 10 M A, 100 mL of 2.00 x 10 M B, 10.0 mL of 1.0 MC and 75 mL of 1.50 x 10 M D. At equilibrium, the concentration of D was measured to be 6.0 x 10 M. a) Calculate the equilibrium concentrations of each species I b) Calculate the equilibrium constant.
Chapter12: Spectrochemical Methods
Section: Chapter Questions
Problem 3P
Related questions
Question
Transcribed Image Text:Q Search the web..
Search
Bb WordPerfect Office Document
ps://blackboard.liu.edu/bbcswebdav/pid-3293771-dt-content-rid-40608391 2/courses/CHM_4_01L_BK_SPRG2022/EXPT%2016%20SPETROP...
2. Consider the reaction:
A + B = C+3D
A solution was prepared by mixing 50.00 mL of 1.00 x 103 MA, 100 mL of 2.00 x 10³M B,
10.0 mL of 1.0MC and 75 mL of 1.50 x 10'M D. At equilibrium, the concentration of D was
measured to be 6.0 x 10 M.
a) Calculate the equilibrium concentrations of each species
I
b) Calculate the equilibrium constant.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



