Chemistry 9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Steven S. Zumdahl
1 Chemical Foundations 2 Atoms, Molecules, And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding: General Concepts 9 Covalent Bonding: Orbitals 10 Liquids And Solids 11 Properties Of Solutions 12 Chemical Kinetics 13 Chemical Equilibrium 14 Acids And Bases 15 Acid-base Equilibria 16 Solubility And Complex Ion Equilibria 17 Spontaneity, Entropy, And Free Energy 18 Electrochemistry 19 The Nucleus: A Chemist's View 20 The Representative Elements 21 Transition Metals And Coordination Chemistry 22 Organic And Biological Molecules Chapter9: Covalent Bonding: Orbitals
Chapter Questions Section: Chapter Questions
Problem 1RQ: Why do we hybtidize atomic orbitals to explain the bonding in covalent compounds? What type of bonds... Problem 2RQ: What hybridization is required for central atoms that have a tetrahedral arrangement of electron... Problem 3RQ: Describe the bonding in H2S, CH4, H2CO and HCN using the localized electron model. Problem 4RQ: What hybridization is required for central atoms exhibiting trigonal bipyramidal geometry?... Problem 5RQ: Electrons in bonding molecular orbitals are most likely to be found in the region between the two... Problem 1ALQ: What are molecular orbitals? How do they compare with atomic orbitals? Can you tell by the shape of... Problem 2ALQ: Explain the difference between the and MOs for homonuclear diatomic molecules. How are bonding and... Problem 3ALQ: Compare Figs. 4-47 and 4-49. Why are they different? Because B2 is known to be paramagnetic, the 2p... Problem 4ALQ: Which of the following would you expect to be more favorable energetically? Explain. a. an H2... Problem 5ALQ: Draw the Lewis structure for HCN. Indicate the hybrid orbitals, and draw a picture showing all the... Problem 6ALQ: Which is the more correct statement: The methane molecule (CH4) is a tetrahedral molecule because it... Problem 7ALQ: Compare and contrast the MO model with the local electron model. When is each useful? Problem 8ALQ: What are the relationships among bond order, bond energy, and bond length? Which of these quantities... Problem 9Q: In the hybrid orbital model, compare and contrast bonds with bonds. What orbitals form the bonds... Problem 10Q: In the molecular orbital mode l, compare and contrast bonds with bonds. What orbitals form the ... Problem 11Q: Why are d orbitals sometimes used to form hybrid orbitals? Which period of elements does not used... Problem 12Q: The atoms in a single bond can rotate about the internuclear axis without breaking the bond. The... Problem 13Q: Compare and contrast bonding molecular orbitals with antibonding molecular orbitals. Problem 14Q: What modification to the molecular orbital model was made from the experimental evidence that B2 is... Problem 15Q: Why does the molecular orbital model do a better job in explaining the bonding in NO and NO than the... Problem 16Q: The three NO bonds in NO3 are all equivalent in length and strength. How is this explained even... Problem 17E: Use the localized electron model to describe the bonding in H2O. Problem 18E: Use the localized electron model to describe the bonding in CCl4. Problem 19E: Use the localized electron model to describe the bonding in H2CO (carbon is the central atom). Problem 20E: Use the localized electron model to describe the bonding in C2H2 (exists as HCCH). Problem 21E: The space-filling models of ethane and ethanol are shown below. Use the localized electron model to... Problem 22E: The space-filling models of hydrogen cyanide and phosgene are shown below. Use the localized... Problem 23E: Give the expected hybridization of the central atom for the molecules or ions in Exercises 81 and 87... Problem 24E: Give the expected hybridization of the central atom for the molecules or ions in Exercises 82 and 88... Problem 25E: Give the expected hybridization of the central atom for the molecules in Exercises 21 and 22. Problem 26E: Give the expected hybridization of the central atom for the molecules in Exercises 27 and 28. Problem 29E: For each of the following molecules, write the Lewis structure(s), predict the molecular structure... Problem 30E: For each of the following molecules or ions that contain sulfur, write the Lewis structure(s),... Problem 31E Problem 32E: The allene molecule has the following Lewis structure: Must all hydrogen atoms lie the same plane?... Problem 33E: Indigo is the dye used in coloring blue jeans. The term navy blue is derived from the use of indigo... Problem 34E: Urea, a compound formed in the liver, is one of the ways humans excrete nitrogen. The Lewis... Problem 35E: Biacetyl and acetoin are added to margarine to make it taste more like butter. Biacetyl Acetion... Problem 36E: Many important compounds in the chemical industry are derivatives of ethylene (C2H4). two of them... Problem 37E: Two molecules used in the polymer industry are azodicarbonamide and methyl cyanoacrylate. Their... Problem 38E: Hot and spicy foods contain molecules that stimulate paindetecting nerve endings. Two such molecules... Problem 39E: One of the first drugs to be approved for use in treatment of acquired immune deficiency syndrome... Problem 40E: The antibiotic thiarubin-A was discovered by studying the feeding habits of wild chimpanzees in... Problem 41E: Consider the following molecular orbitals formed from the combination of two hydrogen 1s orbitals:... Problem 42E: Sketch the molecular orbital and label its type ( or , bonding or antibonding) that would be formed... Problem 43E: Which of the following are predicted by the molecular orbital model to be stable diatomic species?... Problem 44E: Which of the following are predicted by the molecular orbital model to be stable diatomic species?... Problem 45E: Using the molecular orbital model, write electron configurations for the following diatomic species... Problem 46E: Consider the following electron configuration: (3s)2(3s)2(3p)2(3p)4(3p)4 Give four species that, in... Problem 47E: Using molecular orbital theory, explain why the removal of one electron in O2 strengthens bonding,... Problem 48E: Using the molecular orbital model to describe the bonding in F2+, F2, and F2, predict the bond... Problem 49E: The transport of O2 in the blood is carried out by hemoglobin. Carbon monoxide (CO) can interfere... Problem 50E: A Lewis structure obeying the octet rule can be drawn for O2 as follows: Use the molecular orbital... Problem 51E: Using the molecular orbital model, write electron configurations for the following diatomic species... Problem 52E: Using the molecular orbital model, write electron configurations for the following diatomic species... Problem 53E: In which of the following diatomic molecules would the bond strength be expected to weaken as an... Problem 54E: In terms of the molecular orbital model, which species in each of the following two pairs will roost... Problem 55E: Show how two 2p atomic orbitals can combine to form a or a molecular orbital. Problem 56E: Show how a hydrogen 1s atomic orbital and a fluorine 2p atomic orbital overlap to form bonding and... Problem 57E: Use Figs. 4-54 and 4-55 to answer the following questions. a. Would the bonding molecular orbital in... Problem 59E: Acetylene (C2H2) can be produced from the reaction of calcium carbide (CaC2) with water. Use both... Problem 60E: Describe the bonding in NO+, NO, and NO, using both the localized electron and molecular orbital... Problem 61E: Describe the bonding in the O3 molecule and the NO2 ion, using the localized electron model. How... Problem 62E: Describe the bonding in the CO32 ion using the localized electron model. How would the molecular... Problem 63AE: Draw the Lewis structures, predict the molecular structures, and describe the bonding (in terms of... Problem 64AE: FClO2 and F3ClO can both gain a fluoride ion to form stable anions. F3ClO and F3ClO2 will both lose... Problem 65AE: Two structures can be drawn for cyanuric acid: a. Are these two structures the same molecule?... Problem 66AE: Give the expected hybridization for the molecular structures illustrated in the previous question. Problem 67AE: Vitamin B6 is an organic compound whose deficiency in the human body can cause apathy, irritability,... Problem 68AE: Aspartame is an artificial sweetener marketed under the name Nutra-Sweet. A partial Lewis structure... Problem 69AE Problem 70AE: The three most stable oxides of carbon are carbon monoxide (CO), carbon dioxide (CO2), and carbon... Problem 72AE: Complete the following resonance structures for POCl3. a. Would you predict the same molecular... Problem 73AE Problem 74AE: Describe the bonding in the first excited state of N2 (the one closest in energy to the ground... Problem 75AE: Using an MO energy-level diagram, would you expect F2 to have a lower or higher first ionization... Problem 76AE: Show how a dxz. atomic orbital and a pz, atomic orbital combine to form a bonding molecular orbital.... Problem 77AE: What type of molecular orbital would result from the in-phase combination of the two dxz atomic... Problem 78AE: Consider three molecules: A, B, and C. Molecule A has a hybridization of sp3 Molecule B has two more... Problem 80CWP: Draw the Lewis structures for TeCl4, ICl5, PCl5, KrCl4, and XeCl2. Which of the compounds exhibit at... Problem 81CWP: A variety of chlorine oxide fluorides and related cations and anions are known. They tend to be... Problem 82CWP: Pelargondin is the molecule responsible for the red color of the geranium flower. It also... Problem 83CWP: Complete a Lewis structure for the compound shown below, then answer the following questions. What... Problem 84CWP: Which of the following statements concerning SO2 is(are) true? a. The central sulfur atom is sp2... Problem 85CWP: Consider the molecular orbital electron configurations for N2, N2+, and N2. For each compound or... Problem 86CWP: Place the species B2+ , B2, and B2 in order of increasing bond length and increasing bond energy. Problem 87CP: Consider the following computer-generated model of caffeine: Complete a Lewis structure for caffeine... Problem 88CP: Cholesterol (C27liu;O) has the following structure: In such shorthand structures, each point where... Problem 89CP: Cyanamide (H2NCN), an important industrial chemical, is produced by the following steps: Calcium... Problem 91CP: A flask containing gaseous N2 is irradiated with 25-nm light. a. Using the following information,... Problem 92CP Problem 93CP: Values of measured bond energies may vary greatly depending on the molecule studied. Consider the... Problem 94CP: Use the MO model to explain the bonding in BeH2. When constructing the MO energy-level diagram,... Problem 95CP Problem 96CP: Arrange the following from lowest to highest ionization energy: O, O2, O2 , O2+. Explain your... Problem 97CP: Use the MO model to determine which of the following has the smallest ionization energy: N2, O2,... Problem 98CP: Given that the ionization energy of F2 is 290 kJ/mol, do the following: a. Calculate the bond energy... Problem 99CP: Carbon monoxide (CO) forms bonds to a variety of metals and metal ions. liS ability to bond to iron... Problem 100CP Problem 101IP: As the bead engineer of your starship in charge of the warp drive, you notice that the supply of... Problem 103IP: Determine the molecular structure and hybridization of the central atom X in the polyatomic ion XY3+... Problem 82CWP: Pelargondin is the molecule responsible for the red color of the geranium flower. It also...
Related questions
Hi, this is organic chemistry . Thanks for the help, i would really appreciate if you explain it a little bit why the answer would be like it.
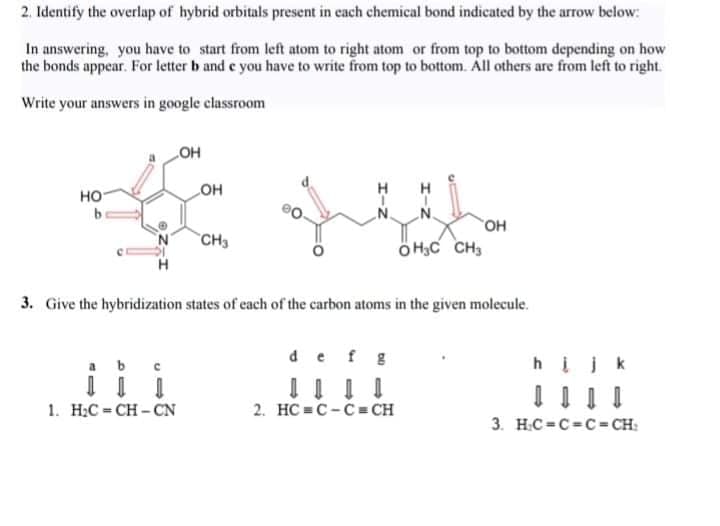
Transcribed Image Text: 2. Identify the overlap of hybrid orbitals present in each chemical bond indicated by the arrow below:
In answering, you have to start from left atom to right atom or from top to bottom depending on how
the bonds appear. For letter b and e you have to write from top to bottom. Áll others are from left to right.
Write your answers in google classroom
HO
он
но
.N.
N.
HO.
ÖH;C CH,
"CH3
3. Give the hybridization states of cach of the carbon atoms in the given molecule.
def g
a b
hįjk
i i i
1. H;C = CH - CN
2. HC = C-C = CH
3. H.C=C=C= CH:
Branch of chemistry concerned with the study of carbon-based compounds, also known as organic compounds. These compounds form due to carbon's notable potential in forming chemical bonds. Due to the abundance of organic compounds on Earth, organic chemistry is crucial in other scientific disciplines, including materials science and pharmaceutical science.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images