4. Indicate the hybridization of the indicated atoms in the molecule below. No formal charges exist on this molecule, which means lone pair electrons must be inferred by you. Refer to previous page if you forgot. how many pi bonds are present in the molecule shown? -NH OH how many sigma bonds are present in the molecule shown?
4. Indicate the hybridization of the indicated atoms in the molecule below. No formal charges exist on this molecule, which means lone pair electrons must be inferred by you. Refer to previous page if you forgot. how many pi bonds are present in the molecule shown? -NH OH how many sigma bonds are present in the molecule shown?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.43QE: For each of the following molecules, complete the Lewis structure and use the VSEPR model to...
Related questions
Question
100%
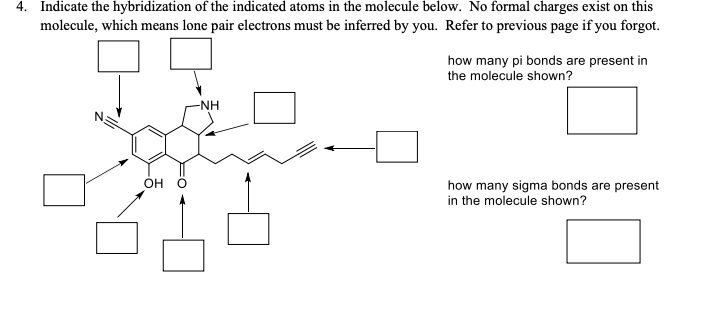
Transcribed Image Text:4.
Indicate the hybridization of the indicated atoms in the molecule below. No formal charges exist on this
molecule, which means lone pair electrons must be inferred by you. Refer to previous page if you forgot.
how many pi bonds are present in
the molecule shown?
-NH
OH
how many sigma bonds are present
in the molecule shown?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning