Now that you have some practice with drawing valence orbital sketches, with and without hybridization, let's practice identifying the types of hybridization and bonding in larger molecules. 2. Stay awake longer: Caffeine is the active ingredient in coffee, tea, and some other beverages. Add lone pairs, as needed, to the below structure of caffeine. Then use it to answer the following questions. A. Determine the electron-domain geometry of each of the atoms labeled a-e. CH3 HC Ne. d. CH3 H3Cb. B. Assign the expected orbital hybridization for each of the atoms labeled a-e. Remember that the number of hybrid orbitals is equal to the number of atomic orbitals that were mixed and equal to the number of electron domains.
Now that you have some practice with drawing valence orbital sketches, with and without hybridization, let's practice identifying the types of hybridization and bonding in larger molecules. 2. Stay awake longer: Caffeine is the active ingredient in coffee, tea, and some other beverages. Add lone pairs, as needed, to the below structure of caffeine. Then use it to answer the following questions. A. Determine the electron-domain geometry of each of the atoms labeled a-e. CH3 HC Ne. d. CH3 H3Cb. B. Assign the expected orbital hybridization for each of the atoms labeled a-e. Remember that the number of hybrid orbitals is equal to the number of atomic orbitals that were mixed and equal to the number of electron domains.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter3: Electron Orbitals
Section: Chapter Questions
Problem 4E: Consider the incomplete orbital representation of O2 , below right. a. Identify which lobes are...
Related questions
Question
This question has only 2 parts not being graded !
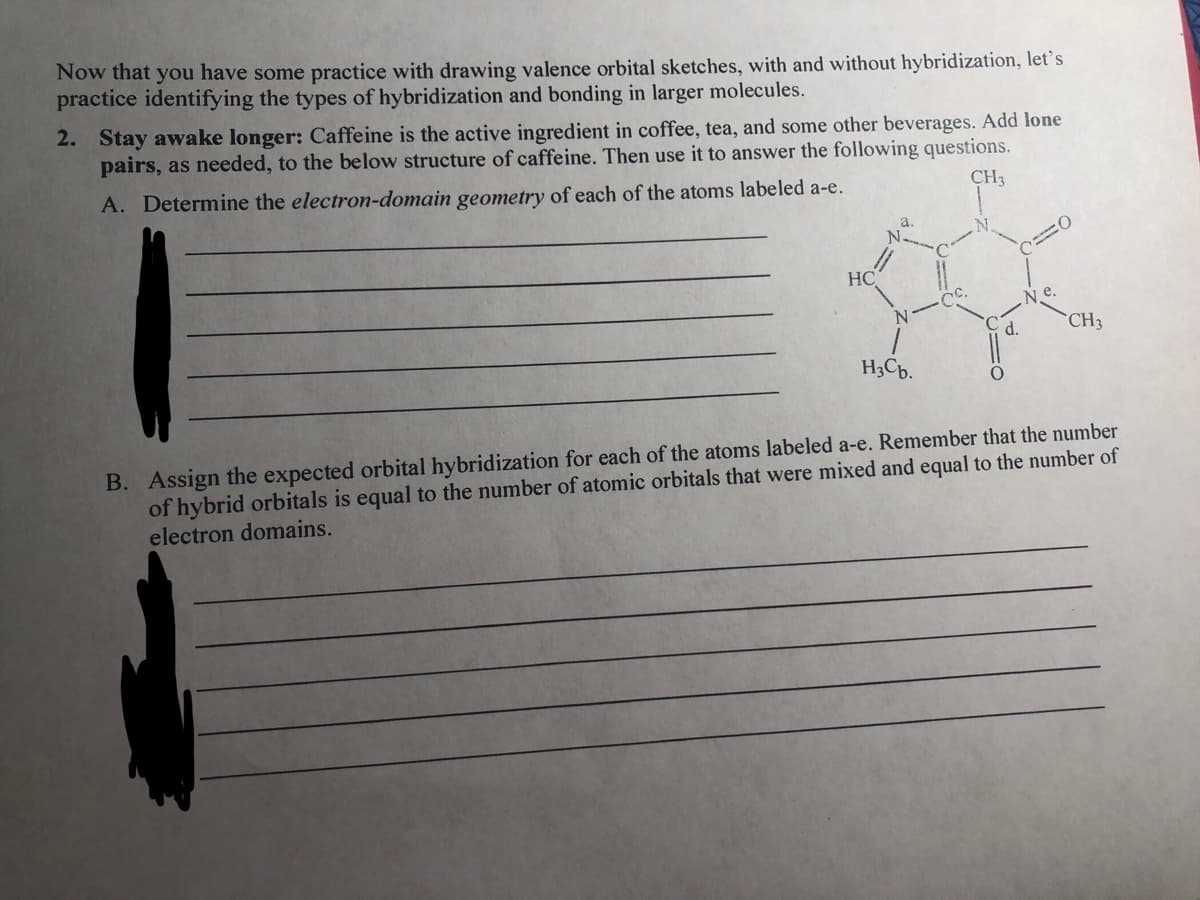
Transcribed Image Text:Now that you have some practice with drawing valence orbital sketches, with and without hybridization, let's
practice identifying the types of hybridization and bonding in larger molecules.
2. Stay awake longer: Caffeine is the active ingredient in coffee, tea, and some other beverages. Add lone
pairs, as needed, to the below structure of caffeine. Then use it to answer the following questions.
A. Determine the electron-domain geometry of each of the atoms labeled a-e.
CH3
HC
N e.
d.
CH3
H3Cb.
B. Assign the expected orbital hybridization for each of the atoms labeled a-e. Remember that the number
of hybrid orbitals is equal to the number of atomic orbitals that were mixed and equal to the number of
electron domains.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning