2. Phoebe was tasked to ensure that an enzyme responsible for keeping her species from extinction remains biologically active. To do so, she needs to store it in a buffer solution with a pH of 10.00. Unfortunately, only three buffer systems are available in their laboratory:
2. Phoebe was tasked to ensure that an enzyme responsible for keeping her species from extinction remains biologically active. To do so, she needs to store it in a buffer solution with a pH of 10.00. Unfortunately, only three buffer systems are available in their laboratory:
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 14P
Related questions
Question
100%
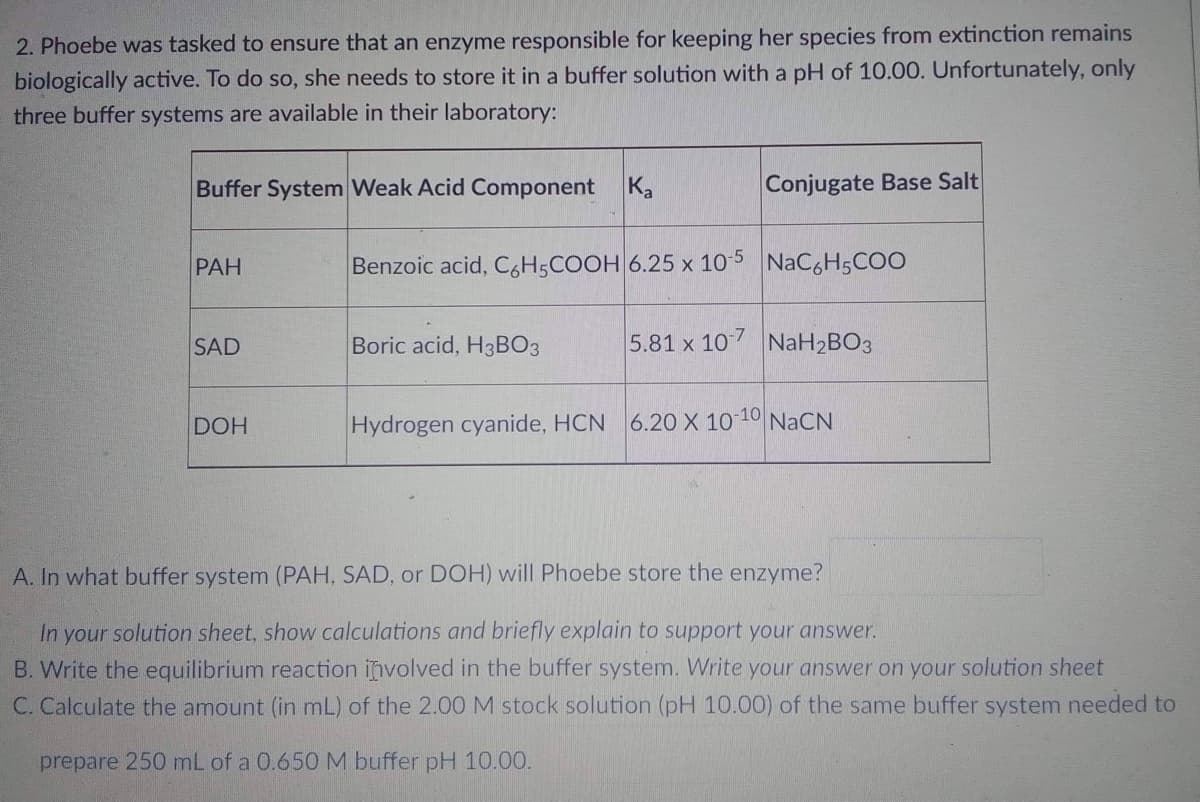
Transcribed Image Text:2. Phoebe was tasked to ensure that an enzyme responsible for keeping her species from extinction remains
biologically active. To do so, she needs to store it in a buffer solution with a pH of 10.00. Unfortunately, only
three buffer systems are available in their laboratory:
Buffer System Weak Acid Component
Ka
Conjugate Base Salt
PAH
Benzoic acid, C6H5COOH 6.25 x 10-5 NaC6H5COO
SAD
Boric acid, H3BO3
5.81 x 10-7 NaH₂BO3
DOH
Hydrogen cyanide, HCN 6.20 X 10-10 NaCN
A. In what buffer system (PAH, SAD, or DOH) will Phoebe store the enzyme?
In your solution sheet, show calculations and briefly explain to support your answer.
B. Write the equilibrium reaction involved in the buffer system. Write your answer on your solution sheet
C. Calculate the amount (in mL) of the 2.00 M stock solution (pH 10.00) of the same buffer system needed to
prepare 250 mL of a 0.650 M buffer pH 10.00.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

