2. Pick 2 more molecules to show how the length of extended carbon-carbon chain affects bp: Longer extended carbon-carbon chain has boiling point a) Draw structures for the two molecules, write the names for all molecules and indicate the IMFs present in each case. bp (1) Sketches: Names: IMFs: bp (2) b) Justify this trend from the point of view of IMFs only. bp (3)
2. Pick 2 more molecules to show how the length of extended carbon-carbon chain affects bp: Longer extended carbon-carbon chain has boiling point a) Draw structures for the two molecules, write the names for all molecules and indicate the IMFs present in each case. bp (1) Sketches: Names: IMFs: bp (2) b) Justify this trend from the point of view of IMFs only. bp (3)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 18E: Under certain conditions, molecules of acetic acid, CH3COOH, form dimers, pairs of acetic acid...
Related questions
Question
How can we complete questions 2-4 with the molecule?

Transcribed Image Text:General Chemistry 1
3. Use the table to predict how the molecular surface area affects boiling point. Think about a
sphere - it has the lowest surface area for the given volume. The closer the molecular shape
to a sphere the lower the surface area. Pick two more molecules from the table with the
similar MM's and similar IMFs but with different surface areas - to illustrate how the surface
area affects bp.
A molecule with a smaller surface area has.
boiling point
a) Draw structures for the two more molecules, name all molecules, and list the types of
intermolecular forces that are present in each case.
bp (1)
Sketches:
Names:_
IMFS:
b) Justify this trend from the point of view of IMFs only.
A.
bp (2)
OH
4. Now that you've looked at all three trends you are ready to compare boiling points!
(a) List the following compounds in order of decreasing boiling points.
B.
OH
C.
D.
(b) Justify your answer using the trends we just discussed.
bp (3)
OH
E.
bp () > bp (_) > bp (_____) > bp () > bp (______)
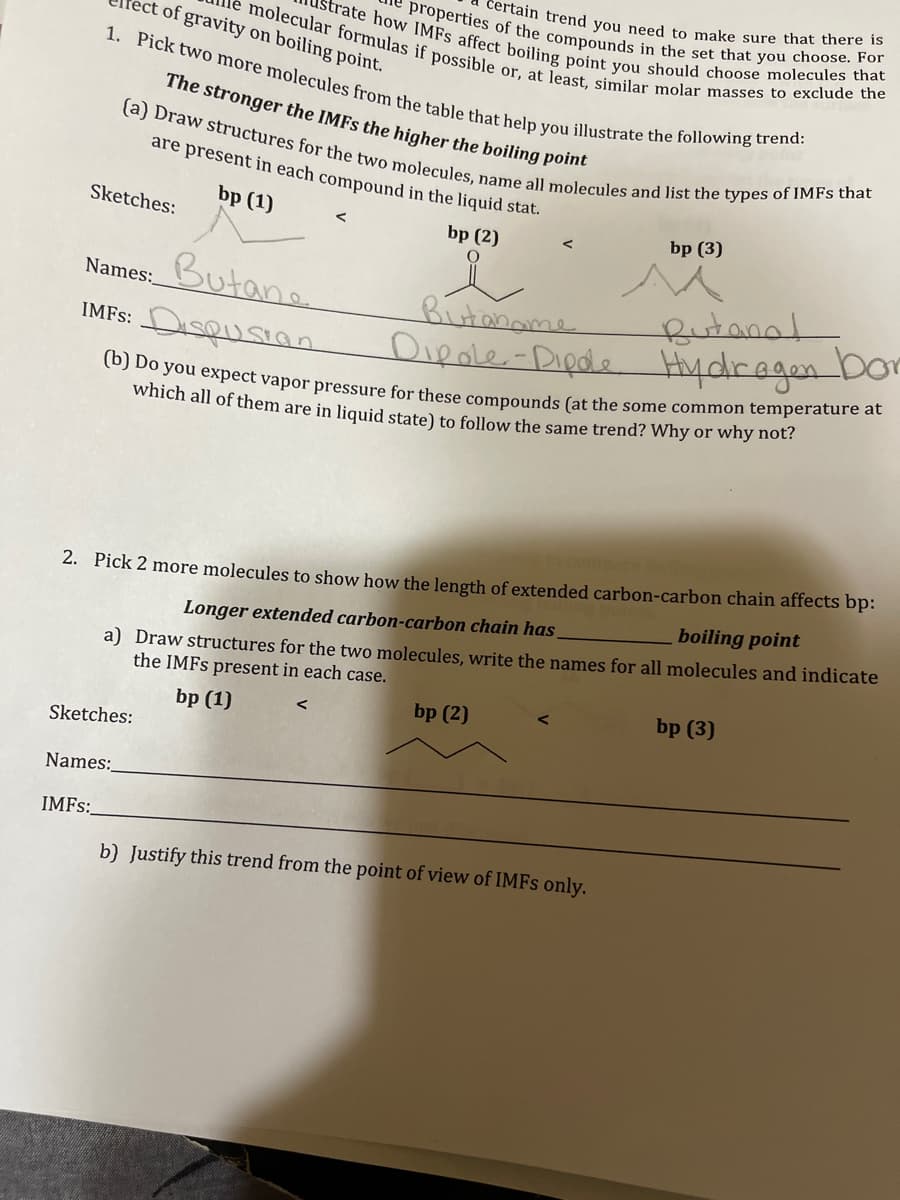
Transcribed Image Text:of gravity on boiling point.
molecular formulas if possible or, at least, similar molar masses to exclude the
strate how IMFS affect boiling point you should choose molecules that
properties of the compounds in the set that you choose. For
1. Pick two more molecules from the table that help you illustrate the following trend:
certain trend you need to make sure that there is
The stronger the IMFs the higher the boiling point
(a) Draw structures for the two molecules, name all molecules and list the types of IMFs that
are present in each compound in the liquid stat.
bp (1)
bp (2)
O
Sketches:
Butane
Names:
IMFS: DISQUSION
Sketches:
Names:
IMFS:
<
Butaname
Dipole-Dipole
(b) Do you expect vapor pressure for these compounds (at the some common temperature at
which all of them are in liquid state) to follow the same trend? Why or why not?
2. Pick 2 more molecules to show how the length of extended carbon-carbon chain affects bp:
Longer extended carbon-carbon chain has
boiling point
a) Draw structures for the two molecules, write the names for all molecules and indicate
the IMFs present in each case.
bp (1)
bp (2)
bp (3)
Butana!
Hydragen bor
b) Justify this trend from the point of view of IMFs only.
bp (3)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning