2. The active component of aspirin is acetylsalicylic acid, HC,H,0., which has a K. of 3.0 x 10-4 (a) Calculate the pH of a solution made by dissolving 8.500 g (500 mg) of acetylsalicylic acid in water and diluting to se.e mL. (b) Repeat the calculation, assuming that the some mass of acetylsalicylic acid is dissolved and diluted to 1900.e mL of solution. (c) Is the pH lower or higher in the mere dilute solution? Which solution hos a higher fraction of its acetylsalicylic acid ionized?
2. The active component of aspirin is acetylsalicylic acid, HC,H,0., which has a K. of 3.0 x 10-4 (a) Calculate the pH of a solution made by dissolving 8.500 g (500 mg) of acetylsalicylic acid in water and diluting to se.e mL. (b) Repeat the calculation, assuming that the some mass of acetylsalicylic acid is dissolved and diluted to 1900.e mL of solution. (c) Is the pH lower or higher in the mere dilute solution? Which solution hos a higher fraction of its acetylsalicylic acid ionized?
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 10RQ: For oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen...
Related questions
Question
How to solve no. 2
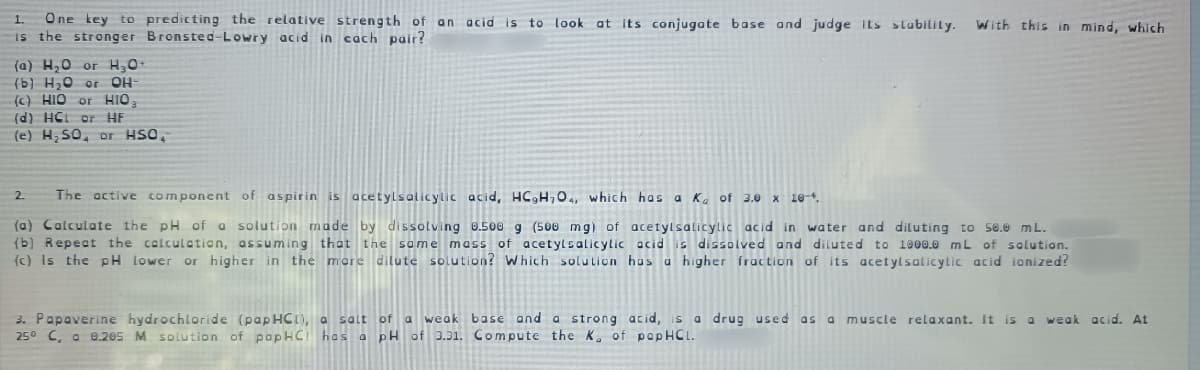
Transcribed Image Text:One key to predicting the relative strength of an acid is to look at its conjugate base and judge its slubility.
is the stronger Bronsted-Lowry acid in cach pair?
1.
With this in mind, which
(a) H,0 or H0
(b) H20 or
OH
(c) HIO or HIO
(d) HCL or HE
(e) H SO, or HSO,
2.
The active component of aspirin is acetylsalicylic acid, HC,H704, which has a Ka of 3.0 x 10-4.
(a) Calculate the pH of a solution made by dissolving 0.500 g (500 mg) of acetylsalicylic acid in water and diluting to s0.0 mL.
(b) Repeat the calculation, assuming that the some mass of acetylsalicylic acid is dissolved and diluted to 1000.0 mL of solution.
(c) Is the pH lower or higher in the mere dilute solution? Which solution has a higher fraction of its acetylsalicylic acid ionized?
3. Papaverine hydrochloride (papHCI), a salt of a weak base and a strong acid, s a drug used as a muscle relaxant. It is a
250 C, a 0.205 M solution of papHCI has a pH of 3.31. Compute the K, of papHCL.
weak acid. At
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning