21. Balance the following equations using half-reaction method: a. As + HNO3 -> H3ASO4 + NO2 in acidic conditions b. Na3CrO3+PbO2+NAOH -> Na2CrO4+Na2PbO2+H2O in basic conditions
21. Balance the following equations using half-reaction method: a. As + HNO3 -> H3ASO4 + NO2 in acidic conditions b. Na3CrO3+PbO2+NAOH -> Na2CrO4+Na2PbO2+H2O in basic conditions
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter20: Chemistry Of The Metals
Section: Chapter Questions
Problem 26QAP
Related questions
Question
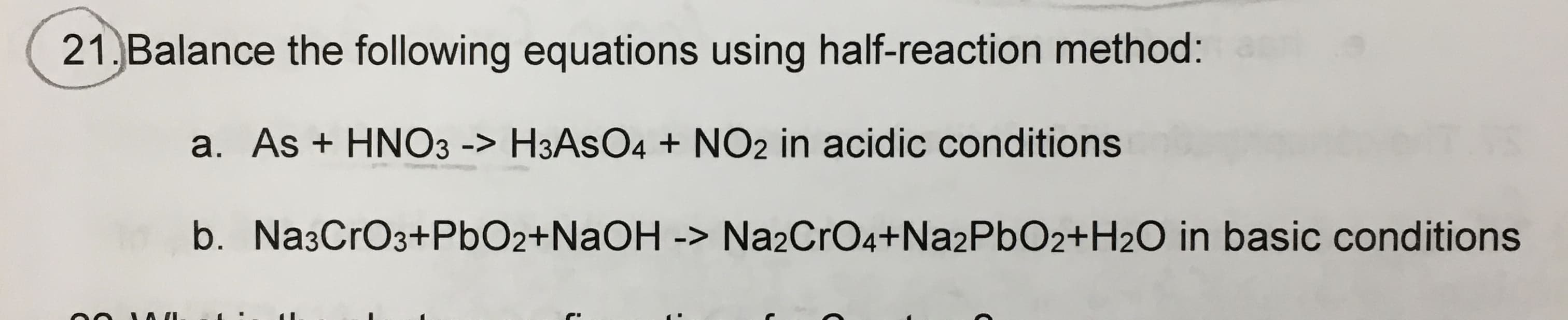
Transcribed Image Text:21. Balance the following equations using half-reaction method:
a. As + HNO3 -> H3ASO4 + NO2 in acidic conditions
b. Na3CrO3+PbO2+NAOH -> Na2CrO4+Na2PbO2+H2O in basic conditions
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps with 10 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
