Given the following Latimer diagram for phosphorous (acidic solution, 25 °C), calculate the potential for the reduction of H4P2O6 to P4 under these conditions. -0.93 +0.38 -0.50 0.51 H,PO, > HP206 H3PO3 H3PO P4 O D. -1.01 V OE-0.63 V O B. -0.01 V O A-1.13 V OC-0.28 V
Given the following Latimer diagram for phosphorous (acidic solution, 25 °C), calculate the potential for the reduction of H4P2O6 to P4 under these conditions. -0.93 +0.38 -0.50 0.51 H,PO, > HP206 H3PO3 H3PO P4 O D. -1.01 V OE-0.63 V O B. -0.01 V O A-1.13 V OC-0.28 V
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter20: Chemistry Of Selected Transition Elements And Coordination Compounds
Section20.5: Chromium
Problem 20.5PSP: At what pH does Ecell = 0.00 V for the reduction of dichromate by iodide ion in acid solution,...
Related questions
Concept explainers
Bond Parameters
Many factors decide the covalent bonding between atoms. Some of the bond parameters are bond angle, bond order, enthalpy, bond length, etc. These parameters decide what kind of bond will form in atoms. Hence it is crucial to understand these parameters in detail and understand how changing these parameters affects the kind of bonding or various characteristics.
Bond Dissociation Energy
The tendency of an atom to attract an electron is known as its electronegativity.
Question
Question in picture
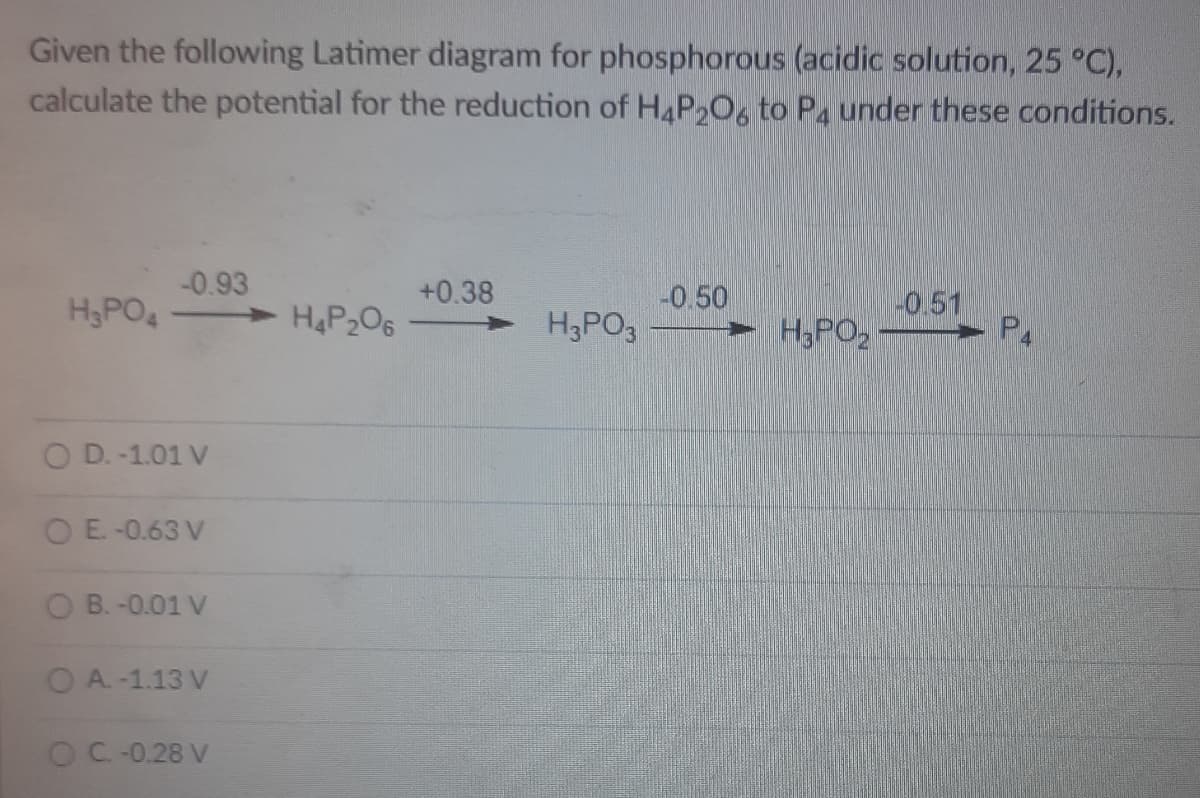
Transcribed Image Text:Given the following Latimer diagram for phosphorous (acidic solution, 25 °C),
calculate the potential for the reduction of H4P2O6 to P4 under these conditions.
-0.93
+0.38
H,PO4
> H4P206
-0.50
H3PO3
H3PO
0.51
P4
O D. -1.01 V
OE.-0.63 V
B. -0.01 V
O A. -1.13 V
OC-0.28 V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
