23.26 The hydrolysis of succinyl COA to succinate and coenzyme A releases energy, and the phosphorylation of ADP requires energy as shown in the given equations. Energy change succinyl CoA + H,0 succinate + HSCOA -9.4 kcal/mol ADP + HPO,2- АТР + Н,о +7.3 kcal/mol a. Combine the equations and write the coupled reaction. b. Is the coupled reaction energetically favorable? Explain.
23.26 The hydrolysis of succinyl COA to succinate and coenzyme A releases energy, and the phosphorylation of ADP requires energy as shown in the given equations. Energy change succinyl CoA + H,0 succinate + HSCOA -9.4 kcal/mol ADP + HPO,2- АТР + Н,о +7.3 kcal/mol a. Combine the equations and write the coupled reaction. b. Is the coupled reaction energetically favorable? Explain.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter23: Enzymes
Section: Chapter Questions
Problem 23.12P
Related questions
Question
SOURCE: GENERAL, ORGANIC AND BIOLOGICAL CHEMISTRY by Smith 4th Edition
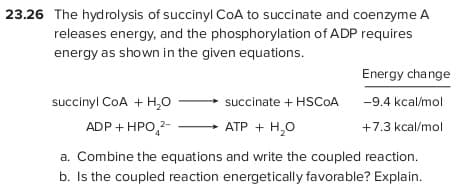
Transcribed Image Text:23.26 The hydrolysis of succinyl COA to succinate and coenzyme A
releases energy, and the phosphorylation of ADP requires
energy as shown in the given equations.
Energy change
succinyl CoA + H,0
succinate + HSCOA
-9.4 kcal/mol
ADP + HPO,2-
АТР + Н,о
+7.3 kcal/mol
a. Combine the equations and write the coupled reaction.
b. Is the coupled reaction energetically favorable? Explain.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning
