. Certain microorganisms with a modified citric acid cycle decarboxylate a- ketoglutarate to produce succinate semialdehyde: COO- Coo- co, CH, CH, CH, CH, C=0 c=0 H a-Ketoglutarate Succinate semialdehyde (a) Succinate semialdehyde is then converted to succinate, which is further metabolized by standard citric acid cycle enzymes. What kind of reaction is required to convert succinate semialdehyde to succinate? Show any coenzymes that might be involved. (b) Based on your answer in part a, how does this pathway compare to the standard citric acid cycle in energy yield?
. Certain microorganisms with a modified citric acid cycle decarboxylate a- ketoglutarate to produce succinate semialdehyde: COO- Coo- co, CH, CH, CH, CH, C=0 c=0 H a-Ketoglutarate Succinate semialdehyde (a) Succinate semialdehyde is then converted to succinate, which is further metabolized by standard citric acid cycle enzymes. What kind of reaction is required to convert succinate semialdehyde to succinate? Show any coenzymes that might be involved. (b) Based on your answer in part a, how does this pathway compare to the standard citric acid cycle in energy yield?
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter23: Carbohydrate Metabolism
Section: Chapter Questions
Problem 23.80E
Related questions
Question

Transcribed Image Text:. Certain microorganisms with a modified citric acid cycle decarboxylate a-
ketoglutarate to produce succinate semialdehyde:
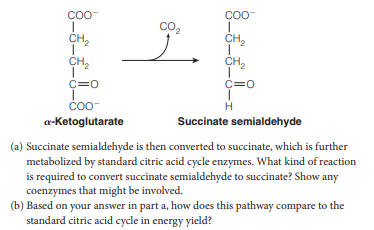
Transcribed Image Text:COO-
Coo-
co,
CH,
CH,
CH,
CH,
C=0
c=0
H
a-Ketoglutarate
Succinate semialdehyde
(a) Succinate semialdehyde is then converted to succinate, which is further
metabolized by standard citric acid cycle enzymes. What kind of reaction
is required to convert succinate semialdehyde to succinate? Show any
coenzymes that might be involved.
(b) Based on your answer in part a, how does this pathway compare to the
standard citric acid cycle in energy yield?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning