27. Using the framework on the right, build the MO energy diagram for CN molecule by assigning C and N atoms to the proper side 2p and filling AO and labeling and filling MO orbitals. 2p B) Write electronic configuration of CN C) What is bond order of CN? D) Is this molecule paramagnetic or diamagnetic 2s 2s E) Arrange CN, CN* and CN' in order of increasing bond energy (weakest – first)
27. Using the framework on the right, build the MO energy diagram for CN molecule by assigning C and N atoms to the proper side 2p and filling AO and labeling and filling MO orbitals. 2p B) Write electronic configuration of CN C) What is bond order of CN? D) Is this molecule paramagnetic or diamagnetic 2s 2s E) Arrange CN, CN* and CN' in order of increasing bond energy (weakest – first)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 30P: The molecular ion HeH+ has an equilibrium bond length of 0.774 Å. Draw an electron correlation...
Related questions
Question
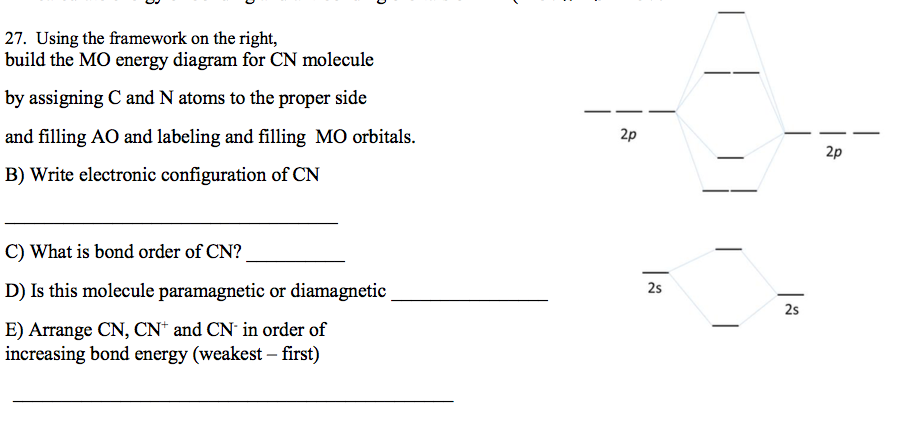
Transcribed Image Text:27. Using the framework on the right,
build the MO energy diagram for CN molecule
by assigning C and N atoms to the proper side
2p
and filling AO and labeling and filling MO orbitals.
2p
B) Write electronic configuration of CN
C) What is bond order of CN?
D) Is this molecule paramagnetic or diamagnetic
2s
2s
E) Arrange CN, CN* and CN' in order of
increasing bond energy (weakest – first)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning