Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.20QAP
Related questions
Question
if the following reaction starts with 28.5 grams of KBr, how many moles of KCI will be produced
Transcribed Image Text:Miracle Askew - 4
ID: 447-656-2882
I Stop Share
Assignments)
om/document/d/12PxnQX-oxdohklZQMnb.
rdson ISD - M.
O Login
AT&T Shopping Cart Apple Music
O (177) FULL QUARA.
M Inbox (1,196) -
A Classes
toichiometry: Molar Ratio w/Mass
TURN IN
- Sha
mat Tools Add-ons Help
Last edit was seconds ago
prmal text
Times New. -
BIUA
12
三, 三
2 3 I 4 T
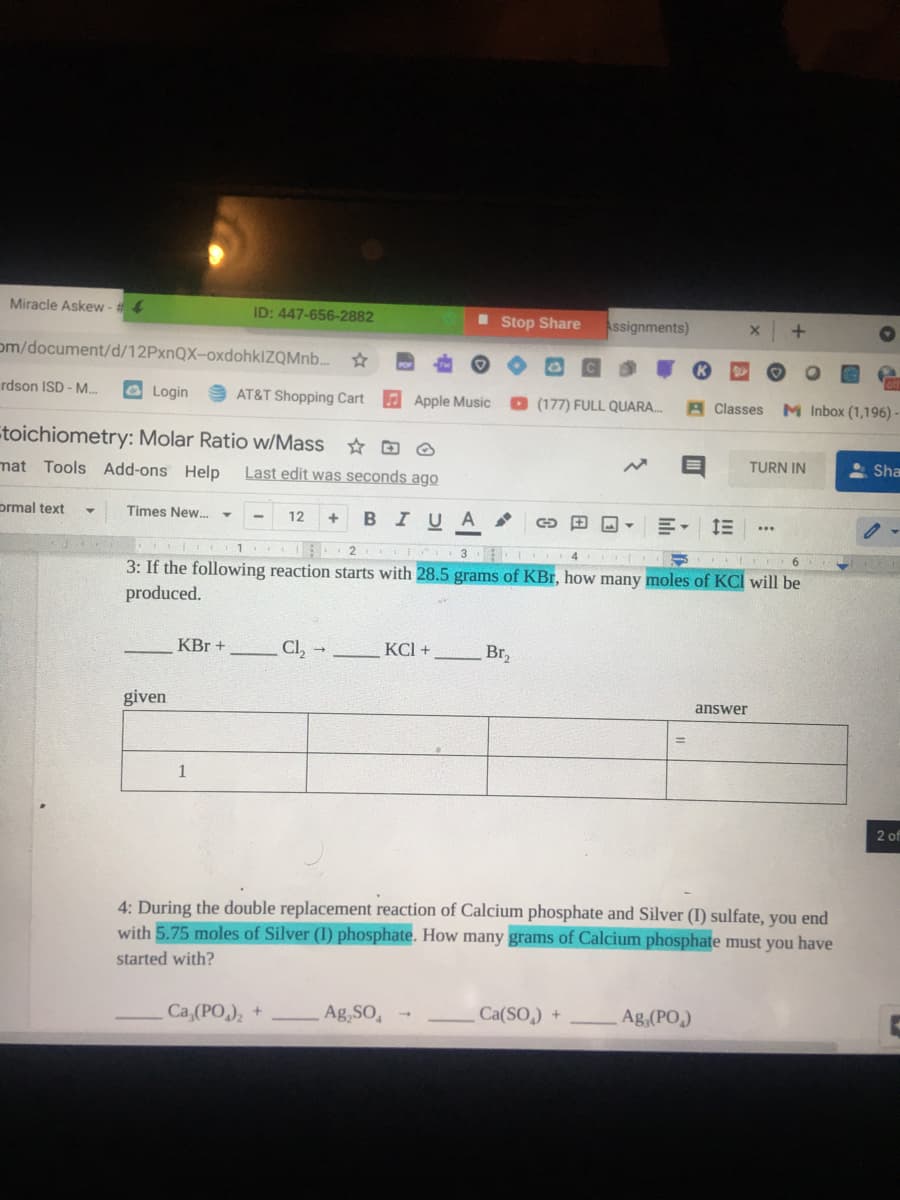
3: If the following reaction starts with 28.5 grams of KBr, how many moles of KCl will be
produced.
KBr +
Cl,
КСI +
Br,
given
answer
1
2 of
4: During the double replacement reaction of Calcium phosphate and Silver (I) sulfate, you end
with 5.75 moles of Silver (I) phosphate. How many grams of Calcium phosphate must you have
started with?
Ca,(PO,), +
Ag,SO,
Ca(SO,) +
Ag,(PO,)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning