3. Hydrochloric acid reacts with aluminum metal to produce hydrogen gas. 2AI (s) + 6HCI (aq) → 2AICI3 (aq) + 3H2 (g) a) How many moles of H2 gas are produced by the reaction of 12.8 g Al metal with excess HCI? Show your work including all unit conversions. Hint: you are going to need the molar mass of Al from periodic table because you have to calculate moles gf Al from grams of Al. Recall mol-to-mol ratio from Unit 2.3 b) How many liters of H2 gas can be collected if the reaction occurs under STP conditions? Show your work. (Recall that STP is 'standard temperature pressure conditions'. Under these conditions, 1 mole of any gas has a volume of 22.4 L)
3. Hydrochloric acid reacts with aluminum metal to produce hydrogen gas. 2AI (s) + 6HCI (aq) → 2AICI3 (aq) + 3H2 (g) a) How many moles of H2 gas are produced by the reaction of 12.8 g Al metal with excess HCI? Show your work including all unit conversions. Hint: you are going to need the molar mass of Al from periodic table because you have to calculate moles gf Al from grams of Al. Recall mol-to-mol ratio from Unit 2.3 b) How many liters of H2 gas can be collected if the reaction occurs under STP conditions? Show your work. (Recall that STP is 'standard temperature pressure conditions'. Under these conditions, 1 mole of any gas has a volume of 22.4 L)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 70QRT
Related questions
Question
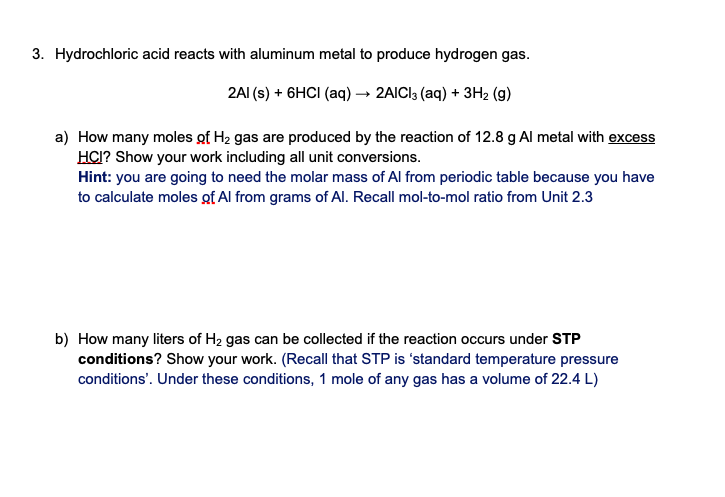
Transcribed Image Text:3. Hydrochloric acid reacts with aluminum metal to produce hydrogen gas.
2AI (s) + 6HCI (aq) → 2AICI3 (aq) + 3H2 (g)
a) How many moles of H2 gas are produced by the reaction of 12.8 g Al metal with excess
HCI? Show your work including all unit conversions.
Hint: you are going to need the molar mass of Al from periodic table because you have
to calculate moles gf Al from grams of Al. Recall mol-to-mol ratio from Unit 2.3
b) How many liters of H2 gas can be collected if the reaction occurs under STP
conditions? Show your work. (Recall that STP is 'standard temperature pressure
conditions'. Under these conditions, 1 mole of any gas has a volume of 22.4 L)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning