3. Some medications permanently inactivate their target enzyme by forming a covalent bond to an amino acid in the enzyme's active site. Aspirin is one example of a pharmaceutical that uses this type of mechanism. So is penicillin. As you saw in the last chapter, the active portion of penicillin is the B-lactam ring. Penicillin forms a covalent bond to serine in the active site of transpeptidase, an enzyme that is essential for forming the bacterial cell wall. Propose a mechanism for the inactivation of transpeptidase by penicillin and show the final product of the reaction. R. NH Он CH3 CH, -N- CH3 backbone
3. Some medications permanently inactivate their target enzyme by forming a covalent bond to an amino acid in the enzyme's active site. Aspirin is one example of a pharmaceutical that uses this type of mechanism. So is penicillin. As you saw in the last chapter, the active portion of penicillin is the B-lactam ring. Penicillin forms a covalent bond to serine in the active site of transpeptidase, an enzyme that is essential for forming the bacterial cell wall. Propose a mechanism for the inactivation of transpeptidase by penicillin and show the final product of the reaction. R. NH Он CH3 CH, -N- CH3 backbone
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter22: Biochemistry
Section: Chapter Questions
Problem 22.3TC
Related questions
Question
100%
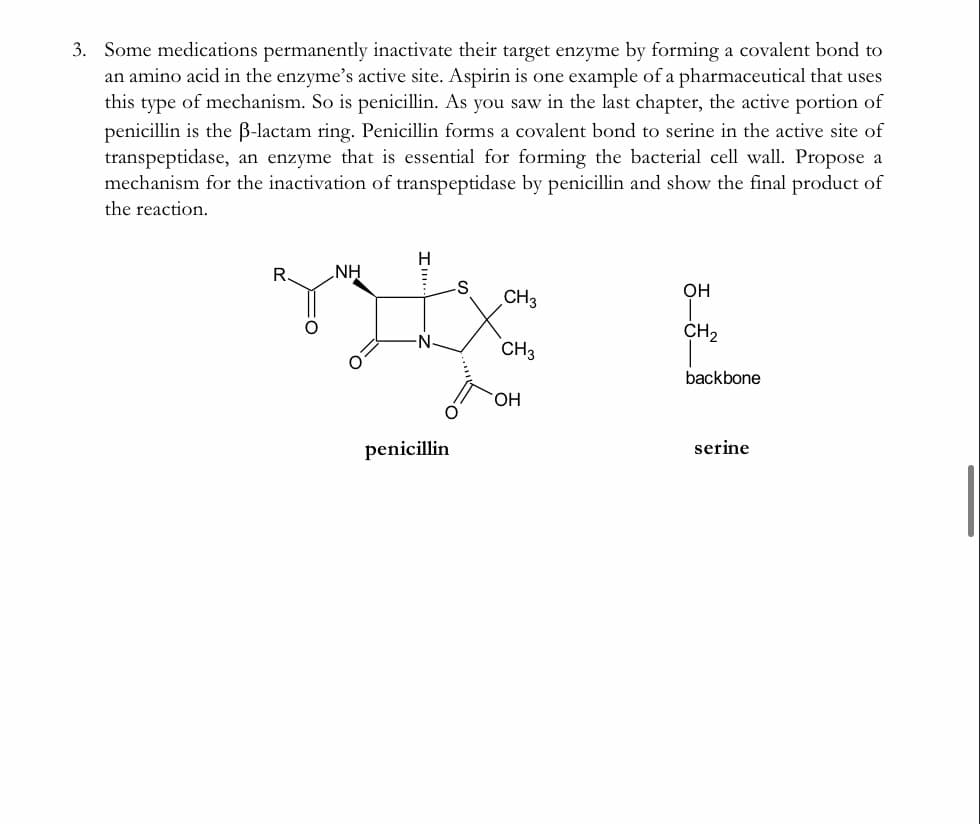
Transcribed Image Text:3. Some medications permanently inactivate their target enzyme by forming a covalent bond to
an amino acid in the enzyme's active site. Aspirin is one example of a pharmaceutical that uses
this type of mechanism. So is penicillin. As you saw in the last chapter, the active portion of
penicillin is the B-lactam ring. Penicillin forms a covalent bond to serine in the active site
transpeptidase, an enzyme that is essential for forming the bacterial cell wall. Propose a
mechanism for the inactivation of transpeptidase by penicillin and show the final product of
the reaction.
NH
OH
CH3
CH2
CH3
backbone
penicillin
serine
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning