1: For the following enzyme catalyzed reaction, indicate where the transition state occurs and whether you expect the enzyme to increase the transition towards the product? Product Reactant Reaction Progression AG Free Energy
1: For the following enzyme catalyzed reaction, indicate where the transition state occurs and whether you expect the enzyme to increase the transition towards the product? Product Reactant Reaction Progression AG Free Energy
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter26: Lipids
Section26.6: Fat-soluble Vitamins
Problem FQ
Related questions
Concept explainers
Organic Chemistry of Metabolic Pathways
Metabolic pathways allude to the arrangement of chemical catalyzed reactions that lead to the transformation of a substance into the final product. Metabolic pathways incorporate a progression of reaction where the substrate is changed continuously and the transitional metabolites are persistently recovered.
Glucogenesis
Glucogenesis is a metabolic pathway in which glucose is produced from carbon substrates that are not carbohydrates. This process is observed in plants, animals, fungi, bacteria and other micro organisms. The general definition for glucogenesis or gluconeogenesis is as follows,
Question
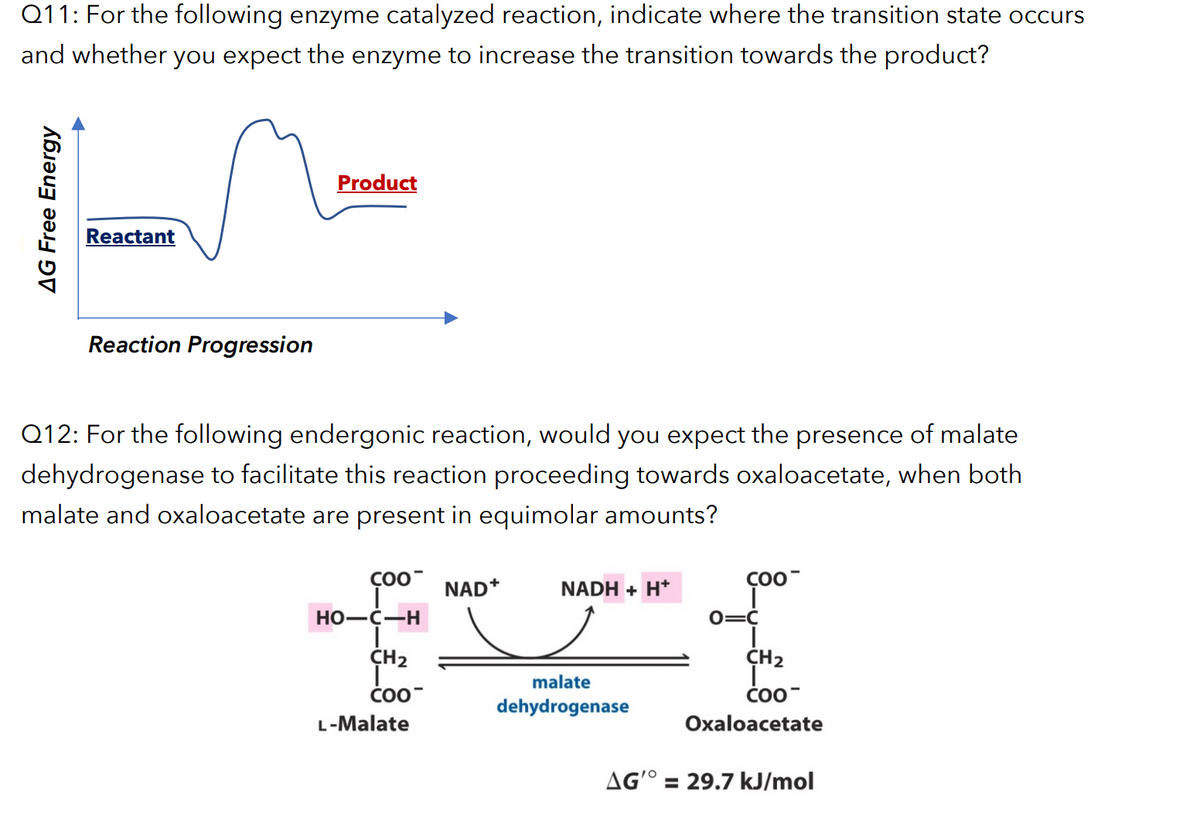
Transcribed Image Text:Q11: For the following enzyme catalyzed reaction, indicate where the transition state occurs
and whether you expect the enzyme to increase the transition towards the product?
Product
Reactant
Reaction Progression
Q12: For the following endergonic reaction, would you expect the presence of malate
dehydrogenase to facilitate this reaction proceeding towards oxaloacetate, when both
malate and oxaloacetate are present in equimolar amounts?
ÇOO-
fo0 NAD*
COO-
NADH + H*
Но—с—Н
0=C
CH2
CH2
malate
CO"
COO-
dehydrogenase
L-Malate
Oxaloacetate
AG'° = 29.7 kJ/mol
AG Free Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning