3. Some values of the rate constant for the alkaline hydrolysis of ethyl iodide over the temperature range 200C-800C are as follows K 10^3 0.100 0.335 1.41 3.06 8.17 21.1 50.1 T/C 20 30 40 50 60 70 80 Calculate the activation energy of the reaction (Ea) and pre-exponential factor (A). (Avery, 50)
3. Some values of the rate constant for the alkaline hydrolysis of ethyl iodide over the temperature range 200C-800C are as follows K 10^3 0.100 0.335 1.41 3.06 8.17 21.1 50.1 T/C 20 30 40 50 60 70 80 Calculate the activation energy of the reaction (Ea) and pre-exponential factor (A). (Avery, 50)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section11.3: Rate Law And Order Of Reactions
Problem 11.5PSP
Related questions
Question
Please help me for number 3
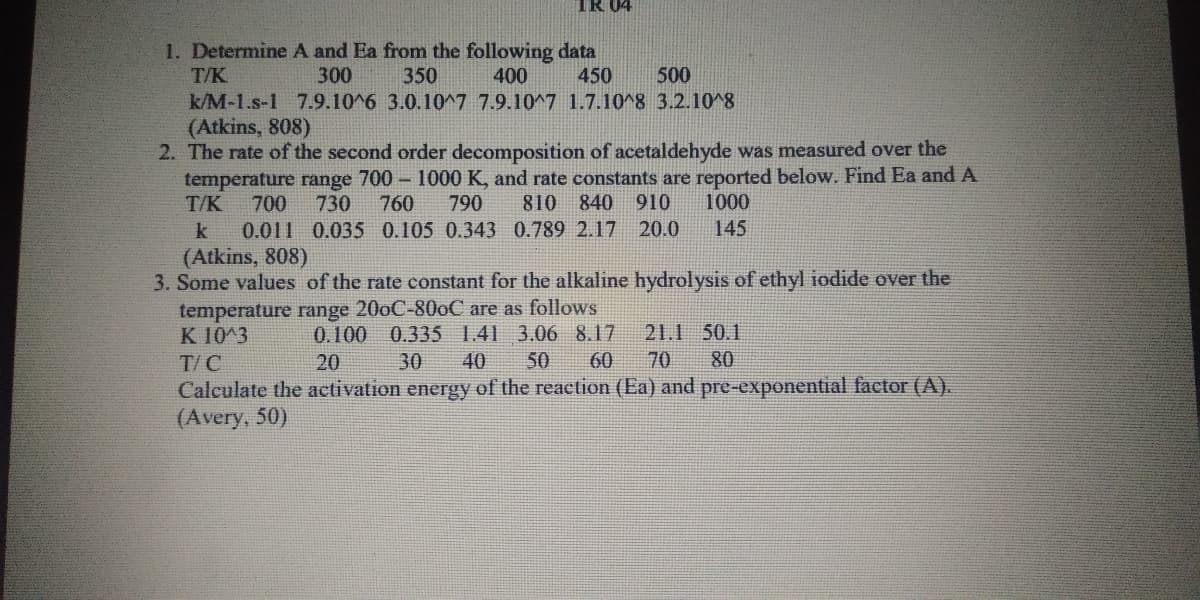
Transcribed Image Text:TR 04
1. Determine A and Ea from the following data
300
T/K
350
400
450
500
k/M-1.s-1 7.9.10^6 3.0.10^7 7.9.10^7 1.7.10^8 3.2.10^8
(Atkins, 808)
2. The rate of the second order decomposition of acetaldehyde was measured over the
temperature range 700 - 1000 K, and rate constants are reported below. Find Ea and A
810
840 910
20.0
T/K
700
730
760
790
1000
k
0.011 0.035 0.105 0.343 0.789 2.17
145
(Atkins, 808)
3. Some values of the rate constant for the alkaline hydrolysis of ethyl iodide over the
temperature range 200C-800C are as follows
0.335 1.41 3.06 8.17
40
K 10^3
0.100
21.1 50.1
T/C
20
30
50
60
70
80
Calculate the activation energy of the reaction (Ea) and pre-exponential factor (A).
(Avery, 50)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
