3. The solubility curves for a molecule, A, in three different solvents are shown below. CH,Cl H.O ELOH 0°C T(°C) BP 0°C T(°C) BP 0°C T("C) BP a. Which solvent should you use for a recrystallization of A? Justify your answer. b. For the other two solvents, explain why the other two solvents would not be appropriate for recrystallization. c. You find that your sample of A contains an impurity, B. On the graph below, sketch the solubility curves for A and B that would produce a pure sample of A from a recrystallization of the impure mixture of A and B. Specify if the impurity is removed during recrystallization or via hot gravity filtration (CH,),CO T("C) BP (TW/3)S
3. The solubility curves for a molecule, A, in three different solvents are shown below. CH,Cl H.O ELOH 0°C T(°C) BP 0°C T(°C) BP 0°C T("C) BP a. Which solvent should you use for a recrystallization of A? Justify your answer. b. For the other two solvents, explain why the other two solvents would not be appropriate for recrystallization. c. You find that your sample of A contains an impurity, B. On the graph below, sketch the solubility curves for A and B that would produce a pure sample of A from a recrystallization of the impure mixture of A and B. Specify if the impurity is removed during recrystallization or via hot gravity filtration (CH,),CO T("C) BP (TW/3)S
Chapter12: Gravimetric Methods Of Analysis
Section: Chapter Questions
Problem 12.1QAP
Related questions
Question
Question attached
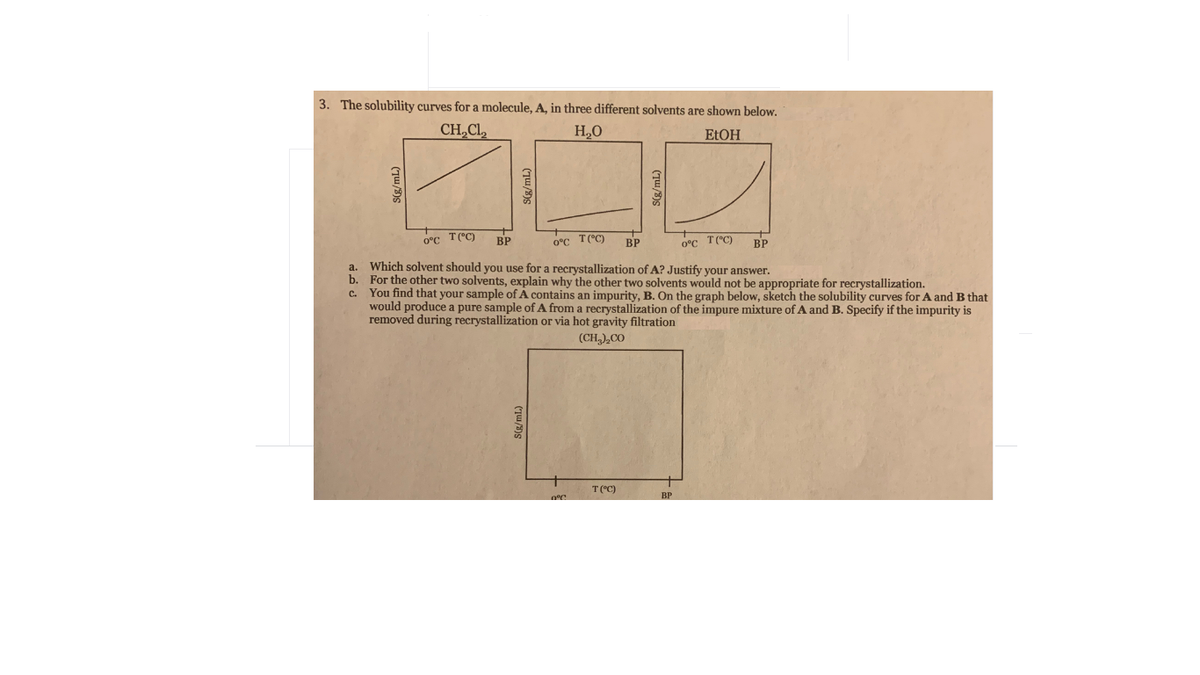
Transcribed Image Text:3. The solubility curves for a molecule, A, in three different solvents are shown below.
CH,Cl
H.O
ELOH
0°C T(°C)
BP
0°C T(°C)
BP
0°C T("C)
BP
a. Which solvent should you use for a recrystallization of A? Justify your answer.
b. For the other two solvents, explain why the other two solvents would not be appropriate for recrystallization.
c. You find that your sample of A contains an impurity, B. On the graph below, sketch the solubility curves for A and B that
would produce a pure sample of A from a recrystallization of the impure mixture of A and B. Specify if the impurity is
removed during recrystallization or via hot gravity filtration
(CH,),CO
T("C)
BP
(TW/3)S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning