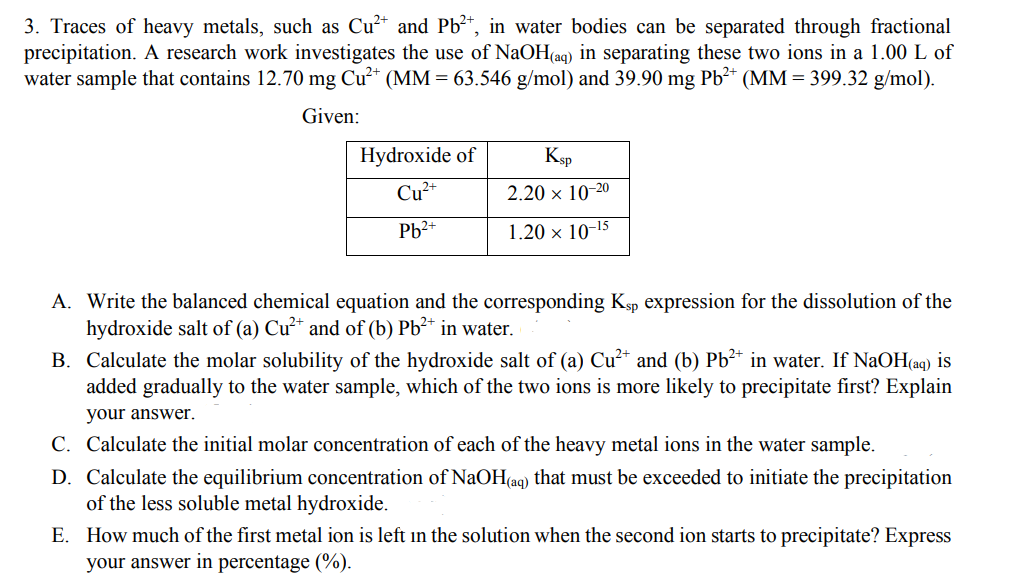
3. Traces of heavy metals, such as Cu²* and Pb²*, in water bodies can be separated through fractional precipitation. A research work investigates the use of NaOH(aq) in separating these two ions in a 1.00 L of water sample that contains 12.70 mg Cu²* (MM = 63.546 g/mol) and 39.90 mg Pb²* (MM = 399.32 g/mol). Given: Hydroxide of Ksp Cu²* 2.20 x 10-20 Pb2+ 1.20 × 10-15 A. Write the balanced chemical equation and the corresponding Ksp expression for the dissolution of the hydroxide salt of (a) Cu²* and of (b) Pb²* in water. B. Calculate the molar solubility of the hydroxide salt of (a) Cu²* and (b) Pb²* in water. If NaOH(aq) is added gradually to the water sample, which of the two ions is more likely to precipitate first? Explain your answer. C. Calculate the initial molar concentration of each of the heavy metal ions in the water sample. D. Calculate the equilibrium concentration of NaOH(aq) that must be exceeded to initiate the precipitation of the less soluble metal hydroxide. E. How much of the first metal ion is left in the solution when the second ion starts to precipitate? Express your answer in percentage (%).
3. Traces of heavy metals, such as Cu²* and Pb²*, in water bodies can be separated through fractional precipitation. A research work investigates the use of NaOH(aq) in separating these two ions in a 1.00 L of water sample that contains 12.70 mg Cu²* (MM = 63.546 g/mol) and 39.90 mg Pb²* (MM = 399.32 g/mol). Given: Hydroxide of Ksp Cu²* 2.20 x 10-20 Pb2+ 1.20 × 10-15 A. Write the balanced chemical equation and the corresponding Ksp expression for the dissolution of the hydroxide salt of (a) Cu²* and of (b) Pb²* in water. B. Calculate the molar solubility of the hydroxide salt of (a) Cu²* and (b) Pb²* in water. If NaOH(aq) is added gradually to the water sample, which of the two ions is more likely to precipitate first? Explain your answer. C. Calculate the initial molar concentration of each of the heavy metal ions in the water sample. D. Calculate the equilibrium concentration of NaOH(aq) that must be exceeded to initiate the precipitation of the less soluble metal hydroxide. E. How much of the first metal ion is left in the solution when the second ion starts to precipitate? Express your answer in percentage (%).
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter7: Chemical Reactions: An Introduction
Section: Chapter Questions
Problem 10A
Related questions
Question
Answer C, D and E only
Transcribed Image Text:3. Traces of heavy metals, such as Cu²* and Pb²*, in water bodies can be separated through fractional
precipitation. A research work investigates the use of NaOH(aq) in separating these two ions in a 1.00 L of
water sample that contains 12.70 mg Cu²* (MM = 63.546 g/mol) and 39.90 mg Pb²* (MM = 399.32 g/mol).
Given:
Hydroxide of
Ksp
Cu2+
2.20 × 10-20
Pb2+
1.20 x 10-15
A. Write the balanced chemical equation and the corresponding Ksp expression for the dissolution of the
hydroxide salt of (a) Cu²* and of (b) Pb²* in water.
B. Calculate the molar solubility of the hydroxide salt of (a) Cu²* and (b) Pb²* in water. If NaOH(aq) is
added gradually to the water sample, which of the two ions is more likely to precipitate first? Explain
your answer.
C. Calculate the initial molar concentration of each of the heavy metal ions in the water sample.
D. Calculate the equilibrium concentration of NaOH(aq) that must be exceeded to initiate the precipitation
of the less soluble metal hydroxide.
E. How much of the first metal ion is left in the solution when the second ion starts to precipitate? Express
your answer in percentage (%).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
