3. Would you expect an alcohol or an ether to have a higher R¡ value on silica gel thin layer chromatography? Why? 4. Draw a resonance structure for the triphenylmethyl cation, showing where else the cationic charge can be delocalized on one of the fully drawn out phenyl rings (Ph). Ph Ph
3. Would you expect an alcohol or an ether to have a higher R¡ value on silica gel thin layer chromatography? Why? 4. Draw a resonance structure for the triphenylmethyl cation, showing where else the cationic charge can be delocalized on one of the fully drawn out phenyl rings (Ph). Ph Ph
Chapter27: Chiral Reduction Of Ethyl Acetoacetate; Optical Purity Determination
Section: Chapter Questions
Problem 1Q
Related questions
Question
Would you expect an alcohol or an ether to have a higher Rf value on silica gel thin layer chromatography? Why?
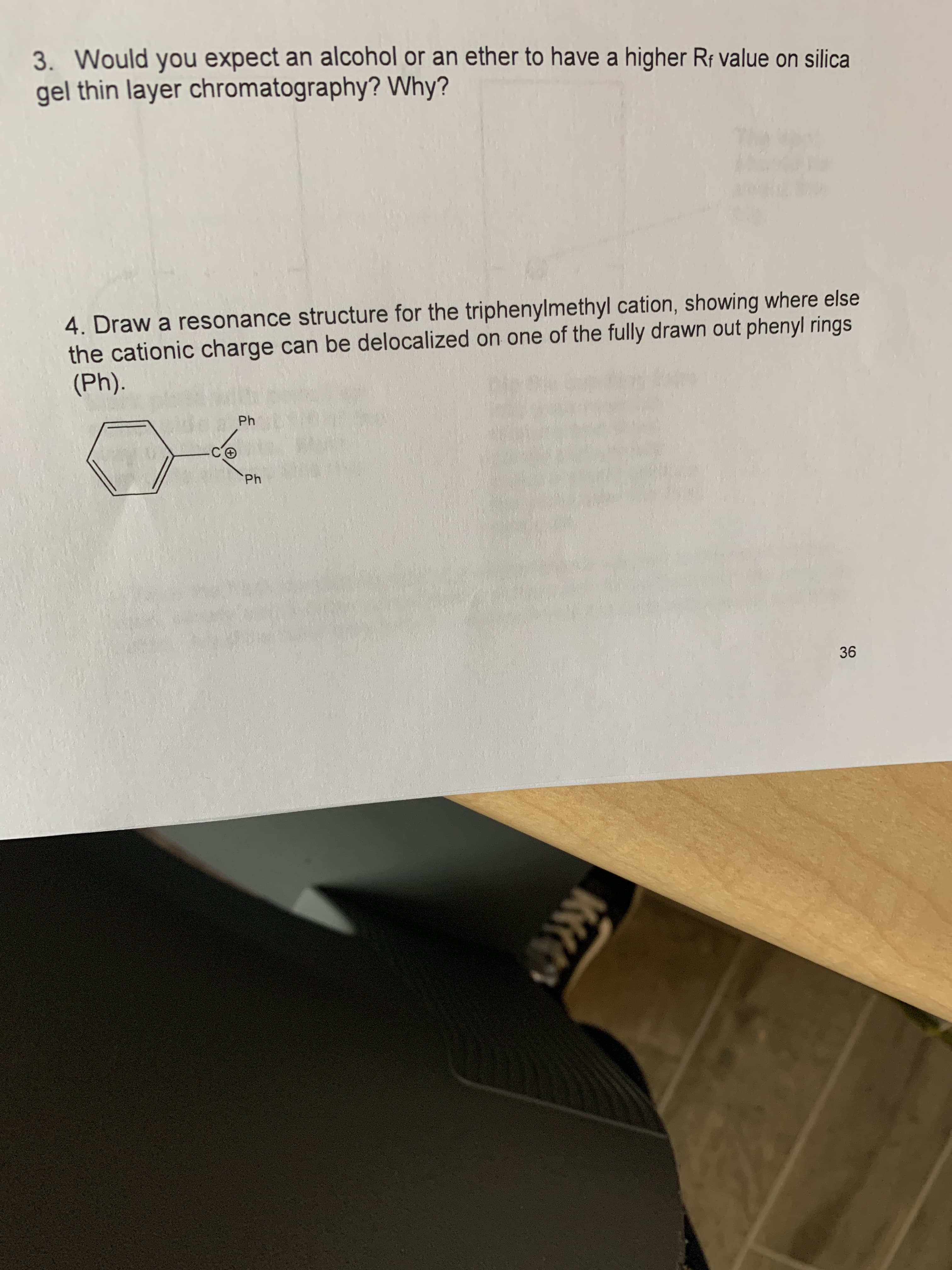
Transcribed Image Text:3. Would you expect an alcohol or an ether to have a higher Rf value on silica
gel thin layer chromatography? Why?
4. Draw a resonance structure for the triphenylmethyl cation, showing where else
the cationic charge can be delocalized on one of the fully drawn out phenyl rings
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole