Choose all that apply.) -In the external circuit, electrons flow from the Ni2+|Ni compartment to the other compartment. -The anode compartment could be Cl2|Cl-. -The anode reaction could be Cr -> Cr3+ + 3e-. -Ni is oxidized at the cathode. -As the cell runs,
Choose all that apply.) -In the external circuit, electrons flow from the Ni2+|Ni compartment to the other compartment. -The anode compartment could be Cl2|Cl-. -The anode reaction could be Cr -> Cr3+ + 3e-. -Ni is oxidized at the cathode. -As the cell runs,
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 76AP
Related questions
Question
Part 1) A standard galvanic cell is constructed in which a Ni2+ | Ni half cell acts as the cathode. Which of the following statements are correct?
(Choose all that apply.)
-In the external circuit, electrons flow from the Ni2+|Ni compartment to the other compartment.
-The anode compartment could be Cl2|Cl-.
-The anode reaction could be Cr -> Cr3+ + 3e-.
-Ni is oxidized at the cathode.
-As the cell runs, anions will migrate from the Ni2+|Ni compartment to the other compartment.
Part 2)A standard galvanic cell is constructed with Zn2+|Zn and Sn2+|Sn half cell compartments connected by a salt bridge. Which of the following statements are correct?
(Choose all that apply.)
-Sn2+ is reduced at the cathode.
-The cathode compartment is the Zn2+|Zn compartment.
-Sn is oxidized at the anode.
-In the external circuit, electrons flow from the Zn2+|Zn compartment to the Sn2+|Sn compartment.
-As the cell runs, anions will migrate from the Zn2+|Zn compartment to the Sn2+|Sn compartment.
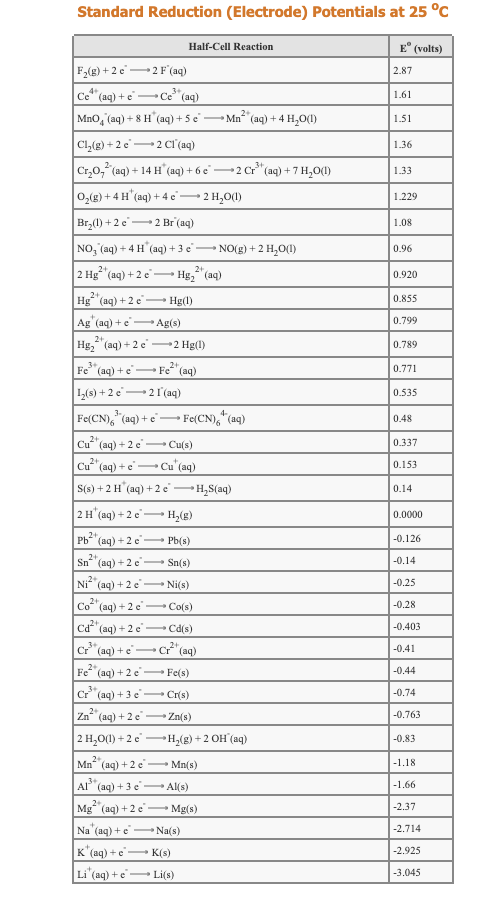
Transcribed Image Text:Standard Reduction (Electrode) Potentials at 25 °c
E° (volts)
Half-Cell Reaction
F2(g) + 2 e2 F' (aq)
2.87
4+
Ce" (aq) + e Ce"(aq)
1.61
-
2+
MnO, (aq) + 8 H" (aq) + 5 e -
Mn (aq) + 4 H,O(1)
1.51
Cl,(g) + 2 e2 CI (aq)
1.36
Cr,0, (aq) + 14 H (aq) + 6 e 2 Cr" (aq) + 7 H,0(1)
0,(g) + 4 H" (aq) + 4 c' 2 H,0(1)
1.33
1.229
Br,(1) + 2 e2 Br (aq)
1.08
NO, (aq) + 4 H (aq) + 3 e NO(g) +2 H,O(1)
0.96
2+
2 H (аg) + 2 e
Hg, (aq)
0.920
2+
0.855
Hg (aq) +2 e Hg(l)
Ag' (aq) + e Ag(s)
0.799
2+
Hg, (aq) + 2 e 2 Hg(1)
0.789
Fe"(aq) +e
• Fe (aq)
0.771
1,(8) + 2 e
21 (aq)
0.535
-
*(aq)
3-
Fe(CN), "(aq) + e
• Fe(CN),
0.48
Cu
(aq) + 2 e Cu(s)
2+
0.337
Cu"(aq) + e Cu"(aq)
0.153
S(s) + 2 H (aq) + 2 e H,S(aq)
0.14
2 H"(aq) + 2 e H,(g)
0.0000
Pb
2+
(aq) +2 e
Pb(s)
-0.126
2+
Sn (aq) + 2 e - Sn(s)
-0.14
Ni" (ag) + 2 e
Ni(s)
-0.25
Co (aq) + 2 e Co(s)
-0.28
.2+
(aq) + 2 e Cd(s)
-0.403
Cr* (aq) + e
- cr (aq)
-0.41
2+
Fe (aq) + 2 e Fe(s)
-0.44
Cr (aq) + 3 e Cr(s)
-0.74
Zn (aq) + 2 e
Zn(s)
-0.763
2 H,0(1) +2 e H,(g) +2 OH (aq)
-0.83
Mn (aq) +2 e
Mn(s)
-1.18
.3+
Al (aq) + 3 e
Al(s)
|-1.66
2
Mg* (aq) +2 e - Mg(s)
-2.37
Na (aq) +e Na(s)
-2.714
K"(aq) +e
Li'(aq) + e
- K(s)
-2.925
Li(s)
-3.045
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
