3.1524 g HCI (FW 36.46) was diluted with 45 mL water and titrated with 1.0149 M NAOH (FW 40) using methyl red as indicator. 28.02 mL of NaOH was required to neutralize the HCI. Calculate the percentage w/w of HCI. Record your answer in decimal notation to 2 decimal places. Answer = % w/w
3.1524 g HCI (FW 36.46) was diluted with 45 mL water and titrated with 1.0149 M NAOH (FW 40) using methyl red as indicator. 28.02 mL of NaOH was required to neutralize the HCI. Calculate the percentage w/w of HCI. Record your answer in decimal notation to 2 decimal places. Answer = % w/w
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
100%
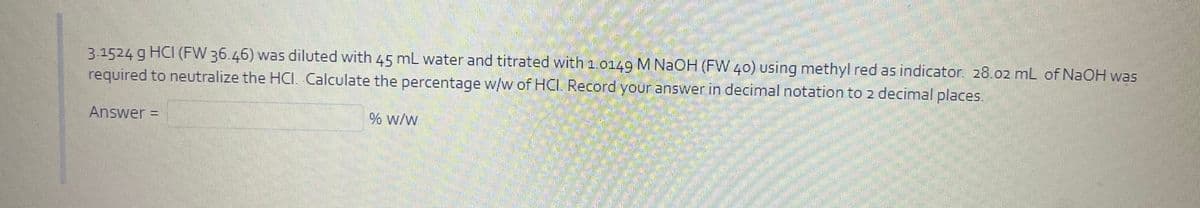
Transcribed Image Text:3.1524 g HCI (FW 36.46) was diluted with 45 mL water and titrated with 1.0149 M NAOH (FW 40) using methyl red as indicator. 28.02 mL of NAOH was
required to neutralize the HCI. Calculate the percentage w/w of HC. Record your answer in decimal notation to 2 decimal places.
Answer =
% w/w
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning