4) The plot below shows the trend in orbital energies for 1s, 2s, 2p, 3s, and 3p orbitals. A more negative potential energy corresponds to a more stable electron in that orbital. Explain the general reasoning behind each of the following trends or observations briefly. Na a Mgo Si Al В 3p -10 2P Şi 1s -20 He 25 Не 3s CI -30 -40 -50 + 10 15 20 Atomic number Miessler, Fischer & Tarr. Inorganic Chemistry 5th Edition. a) For the same value of n and I quantum numbers, an orbital is stabilized by moving to the right of the periodic table (e.g. B →C→N→O...) b) The trend you just described in question part a) has a larger effect on the 2s series than on the 2p series. Potential energy (eV)
4) The plot below shows the trend in orbital energies for 1s, 2s, 2p, 3s, and 3p orbitals. A more negative potential energy corresponds to a more stable electron in that orbital. Explain the general reasoning behind each of the following trends or observations briefly. Na a Mgo Si Al В 3p -10 2P Şi 1s -20 He 25 Не 3s CI -30 -40 -50 + 10 15 20 Atomic number Miessler, Fischer & Tarr. Inorganic Chemistry 5th Edition. a) For the same value of n and I quantum numbers, an orbital is stabilized by moving to the right of the periodic table (e.g. B →C→N→O...) b) The trend you just described in question part a) has a larger effect on the 2s series than on the 2p series. Potential energy (eV)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 26P: Following the pattern of Figure 6.21, work out the correlation diagram for the BeN molecule, showing...
Related questions
Question
Help
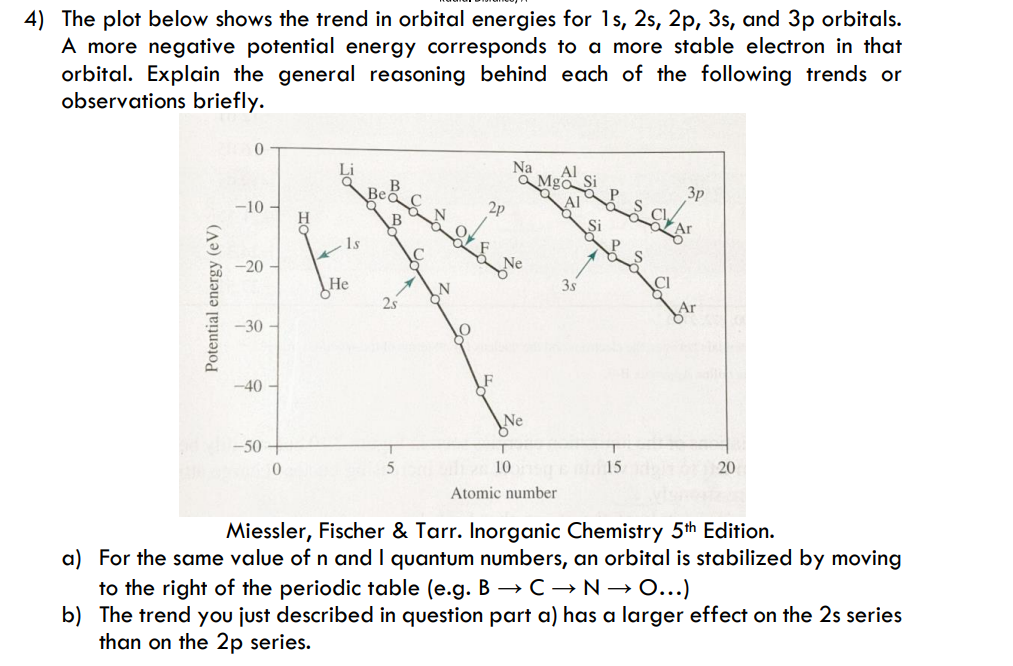
Transcribed Image Text:4) The plot below shows the trend in orbital energies for 1s, 2s, 2p, 3s, and 3p orbitals.
A more negative potential energy corresponds to a more stable electron in that
orbital. Explain the general reasoning behind each of the following trends or
observations briefly.
3p
-10
1s
-20
He
25
35
-30
-40
-50
10
15
20
Atomic number
Miessler, Fischer & Tarr. Inorganic Chemistry 5th Edition.
a) For the same value of n and I quantum numbers, an orbital is stabilized by moving
to the right of the periodic table (e.g. B → C →N→ 0...)
b) The trend you just described in question part a) has a larger effect on the 2s series
than on the 2p series.
Potential energ
energy (eV)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER