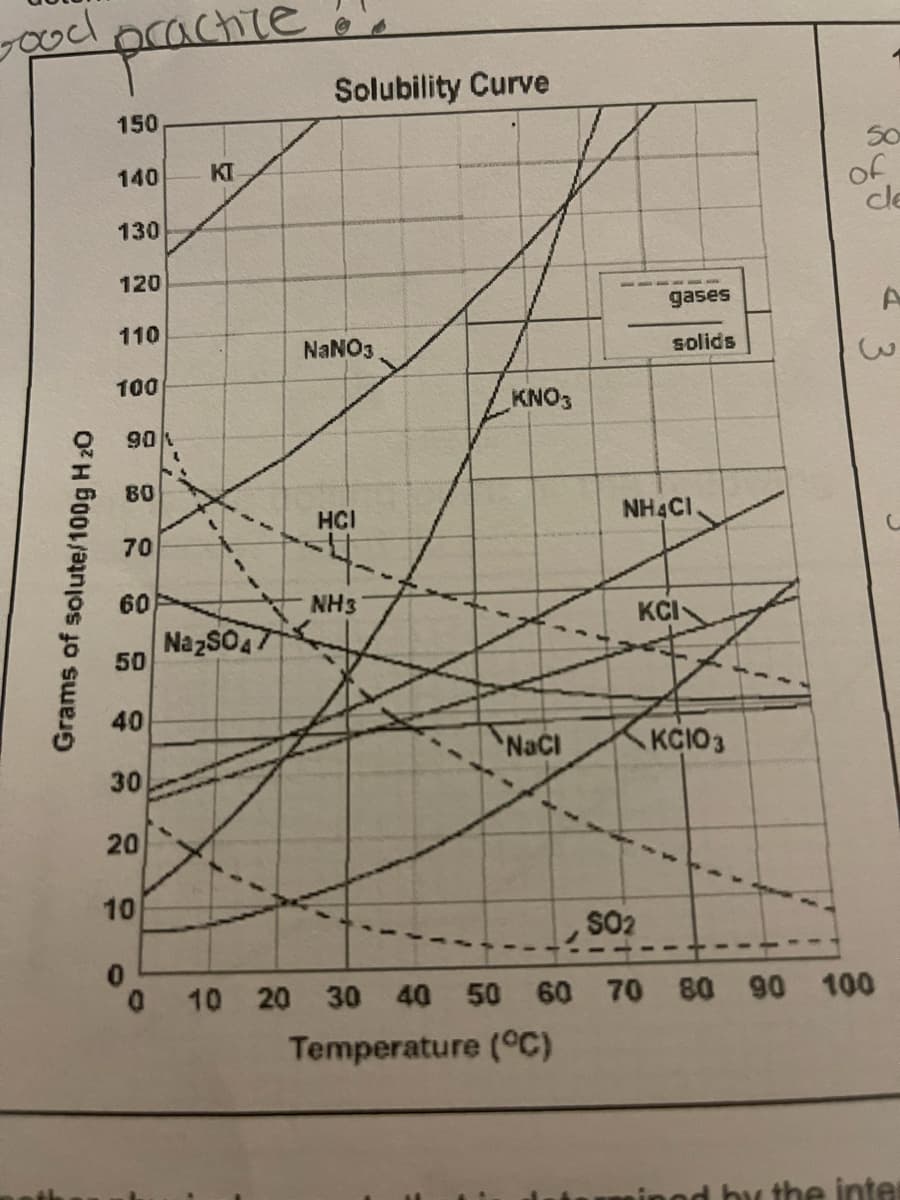
4. How much NaCl will saturate 100 g of water at 50°C? 5. Which of the solids in the graph does temperature seem to have the least effect on its solubility? Explain how you know.
4. How much NaCl will saturate 100 g of water at 50°C? 5. Which of the solids in the graph does temperature seem to have the least effect on its solubility? Explain how you know.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section13.1: Units Of Concentration
Problem 1RC: You dissolve 1.0 mol of urea (H2NCONH2) in 270 g of water. The mole fraction of urea is (a) 3710 3...
Related questions
Question
Transcribed Image Text:y0odocactil(e
Solubility Curve
150
50
KI
of
cle
140
130
120
gases
A
110
NANO3
solids
3)
100
KNO3
90
80
HCI
NH4CI
70
60
NH3
KCI
NazsO47
50
40
NaCI
KCIO3
30
20
SO2
10 20 30 40 50 60 70 80
90 100
Temperature (°C)
ino
by the inte
Grams of solute/100g H 20
10
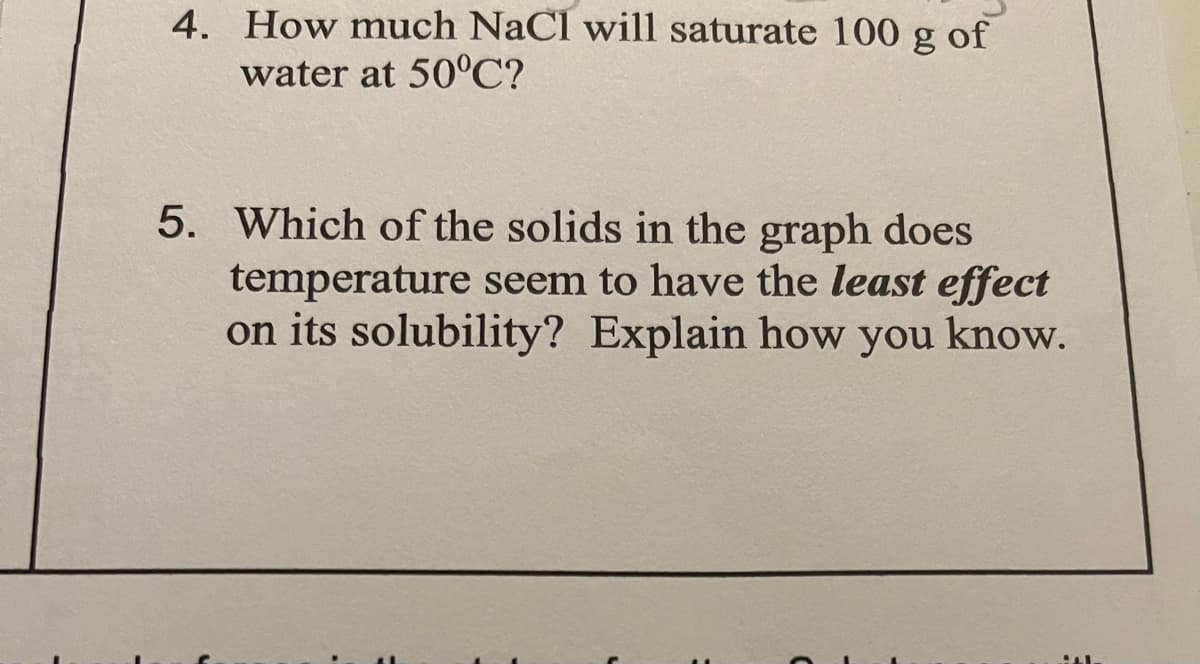
Transcribed Image Text:4. How much NaCl will saturate 100 g of
water at 50C?
5. Which of the solids in the graph does
temperature seem to have the least effect
on its solubility? Explain how you know.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning