5 5 1 1 Part D-Double Replacement Reactions: In this section, it is important to observe the mixtures you make very carefully. You are looking for the formation of either a precipitate or a gas. If the mixture gets cloudy, that is an indication that a precipitate has formed. 1) Get two medium test tubes. Into one, place about 2 mL of a solution of barium chloride. Into the other add about 2 mL of copper(II) sulfate solution. Note the appearances of the two solutions and then mix them together noting any changes that have taken place. Change(s) that took place when the solutions were mixed: Balanced chemical equation: Balanced ionic equation: Balanced net ionic equation: 57 E /177
5 5 1 1 Part D-Double Replacement Reactions: In this section, it is important to observe the mixtures you make very carefully. You are looking for the formation of either a precipitate or a gas. If the mixture gets cloudy, that is an indication that a precipitate has formed. 1) Get two medium test tubes. Into one, place about 2 mL of a solution of barium chloride. Into the other add about 2 mL of copper(II) sulfate solution. Note the appearances of the two solutions and then mix them together noting any changes that have taken place. Change(s) that took place when the solutions were mixed: Balanced chemical equation: Balanced ionic equation: Balanced net ionic equation: 57 E /177
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter6: Chemical Reactions: An Introduction
Section: Chapter Questions
Problem 33QAP: The element tin often occurs in nature as the oxide, SnO2 . To produce pure tin metal from this sort...
Related questions
Question
Transcribed Image Text:31
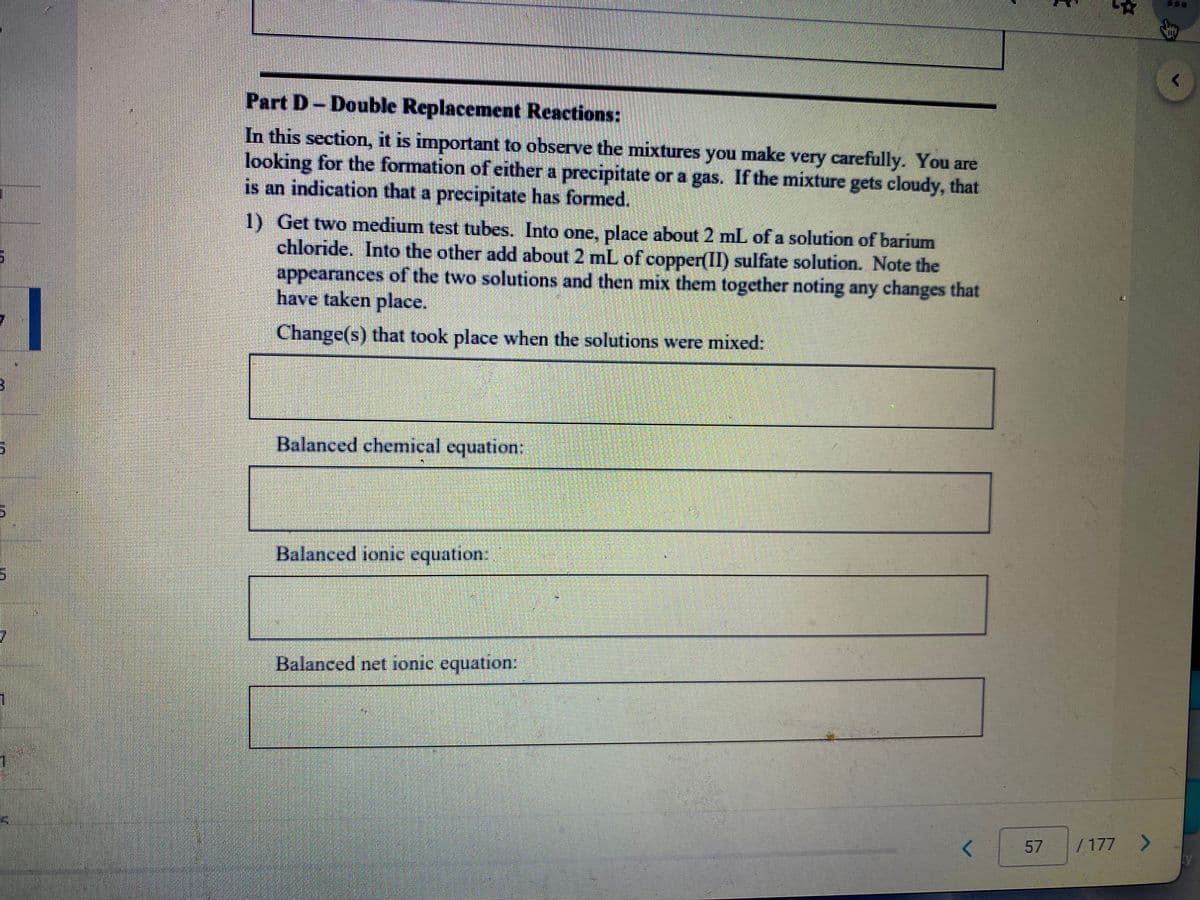
Part D- Double Replacement Reactions:
In this section, it is important to observe the mixtures you make very carefully. You are
looking for the formation of either a precipitate or a gas. If the mixture gets cloudy, that
is an indication that a precipitate has formed.
1) Get two medium test tubes. Into one, place about 2 mL of a solution of barium
chloride. Into the other add about 2 mL of copper(II) sulfate solution. Note the
appearances of the two solutions and then mix them together noting any changes that
have taken place.
Change(s) that took place when the solutions were mixed:
Balanced chemical equation:
Balanced ionic equation:
Balanced net ionic equation:
<
57
*
/177
>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER