5 6 2 8 3 7 9 4 ammonium phosphate 14. Write the correct name or formula for the following. Compound 1 11 4 12 14 16 17 10 aluminum acetate sulfate dihydrogen monoxide selenite 13 potassium bromide silicon dioxide sulfuric acid 15 cobalt(II) nitrite acetic acid 18 manganese(IV) sulfide Formula HC1 CaF₂ CuSO4 H3PO3 C4H10 N₂O HCIO4
5 6 2 8 3 7 9 4 ammonium phosphate 14. Write the correct name or formula for the following. Compound 1 11 4 12 14 16 17 10 aluminum acetate sulfate dihydrogen monoxide selenite 13 potassium bromide silicon dioxide sulfuric acid 15 cobalt(II) nitrite acetic acid 18 manganese(IV) sulfide Formula HC1 CaF₂ CuSO4 H3PO3 C4H10 N₂O HCIO4
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 2.89QE
Related questions
Question
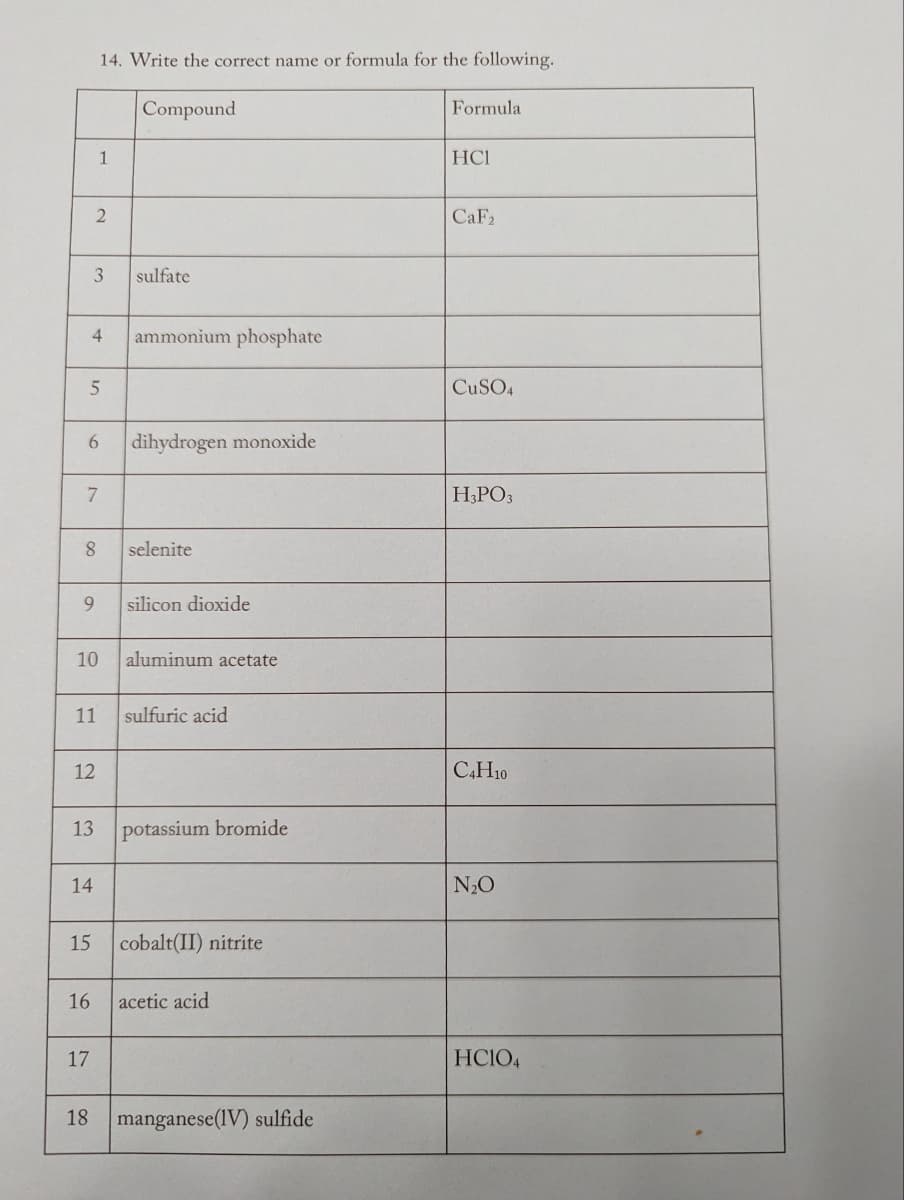
Transcribed Image Text:6
2
5
3
8
4 ammonium phosphate
7
9
14. Write the correct name or formula for the following.
Compound
1
11
12
14
16
17
sulfate
10 aluminum acetate
dihydrogen monoxide
selenite
silicon dioxide
13 potassium bromide
sulfuric acid
15 cobalt(II) nitrite
acetic acid
18 manganese (IV) sulfide
Formula
HCI
CaF2
CuSO4
H3PO3
C4H10
N₂O
HC1O4
Expert Solution
Step 1
Note: Since you have posted a question with multiple sub-parts, we will solve the first three subparts for you. To get the remaining sub-part solved please repost the complete question and mention the sub-parts to be solved.
Ions are charged particles that form when an atom (or group of atoms) gains or loses an electron, and ionic compounds are composed of these charged particles. An anion is a negatively charged ion, whereas a cation is a positively charged ion.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning