5. Formic acid is an organic acid, found in the venom of ants and bees. The diagram shows a molecule of formic acid dissociating in water. || H H H H. H H H. a) Determine the dissociation constant of a 0.100 mol L-' formic acid solution that has a pH of 2.38. b) Use the dissociation constant calculated in part (a) to identify the strength of this acid.
5. Formic acid is an organic acid, found in the venom of ants and bees. The diagram shows a molecule of formic acid dissociating in water. || H H H H. H H H. a) Determine the dissociation constant of a 0.100 mol L-' formic acid solution that has a pH of 2.38. b) Use the dissociation constant calculated in part (a) to identify the strength of this acid.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter13: Acids And Bases
Section: Chapter Questions
Problem 58QAP: Consider citric acid, H3C6H5O7, added to many soft drinks. The equilibrium constants for its...
Related questions
Question
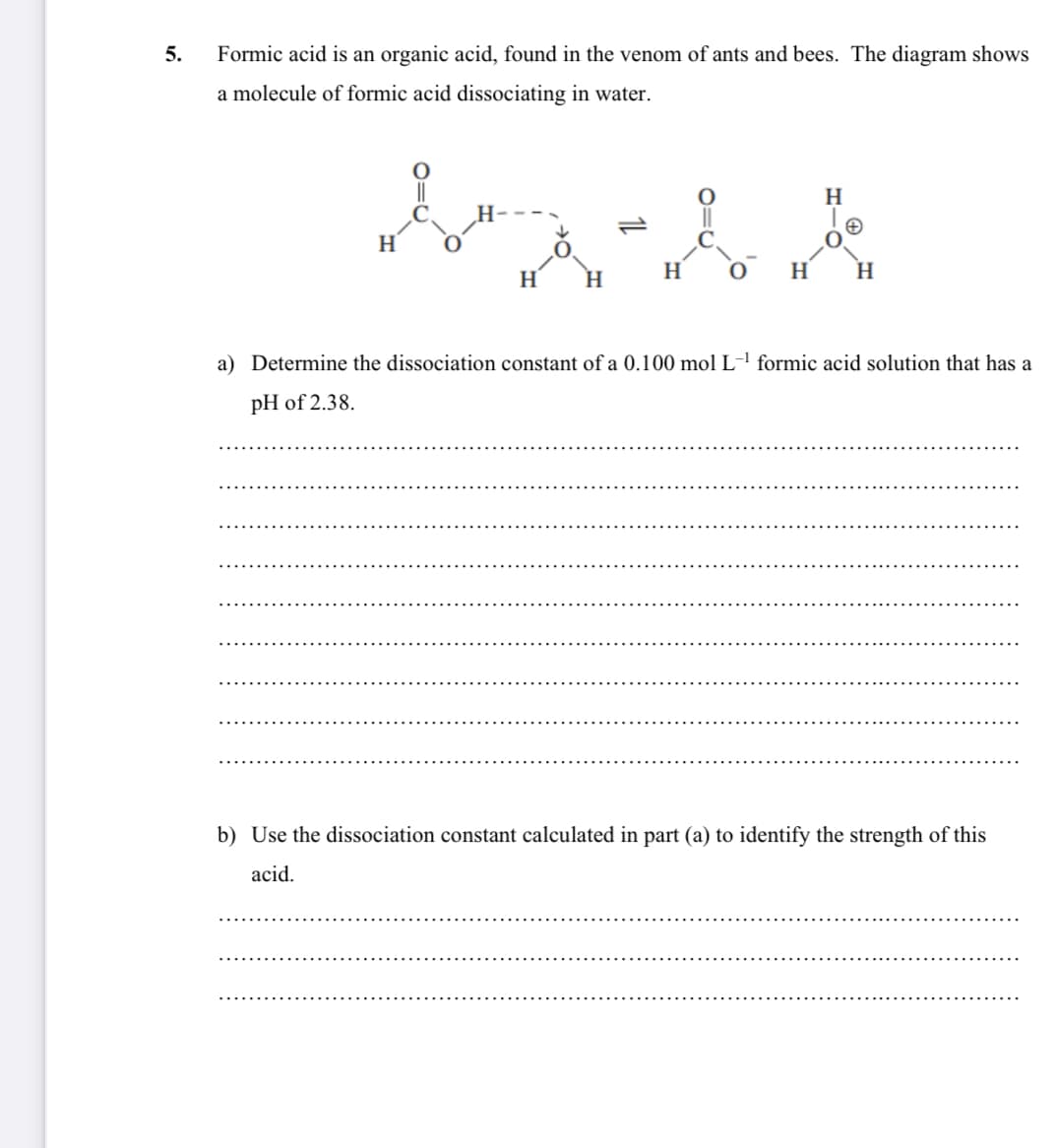
Transcribed Image Text:5.
Formic acid is an organic acid, found in the venom of ants and bees. The diagram shows
a molecule of formic acid dissociating in water.
||
H
H
H
H
H
H
a) Determine the dissociation constant of a 0.100 mol L-' formic acid solution that has a
pH of 2.38.
b) Use the dissociation constant calculated in part (a) to identify the strength of this
acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning