b) Determine the formula of the acid and the formula of the base to which each salt is related and put it them the appropriate columns in Table IV. using A= Acidic Salt, B = Basic Salt, and N = Neutral Salt. a) Copy your classification for each salt in Table III into Table IV. You can abbreviate in the "strength" (i.e. weak or strong) for each acid and base. NaOH + TABLE IV Parent Base Base Strength Salt Parent Acid Acid Strength Class. 0.1 M KC,H,CO, weak 0.1 M CH,NHNO, 0.1 M Sr(NO,)2 2. 0.1 M NaC,H,02 SB KA 0.1 M CH,CH,NH,CI 2. 0.1 M RBCIO 4 0.1 M NHI strong SB 0.1 M NaHCOO 0.1 M KBr strong SB 0.1 M K,S
b) Determine the formula of the acid and the formula of the base to which each salt is related and put it them the appropriate columns in Table IV. using A= Acidic Salt, B = Basic Salt, and N = Neutral Salt. a) Copy your classification for each salt in Table III into Table IV. You can abbreviate in the "strength" (i.e. weak or strong) for each acid and base. NaOH + TABLE IV Parent Base Base Strength Salt Parent Acid Acid Strength Class. 0.1 M KC,H,CO, weak 0.1 M CH,NHNO, 0.1 M Sr(NO,)2 2. 0.1 M NaC,H,02 SB KA 0.1 M CH,CH,NH,CI 2. 0.1 M RBCIO 4 0.1 M NHI strong SB 0.1 M NaHCOO 0.1 M KBr strong SB 0.1 M K,S
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 15E: Mark each of the following statements True or False: a. The conjugate base of a strong acid is...
Related questions
Question
100%
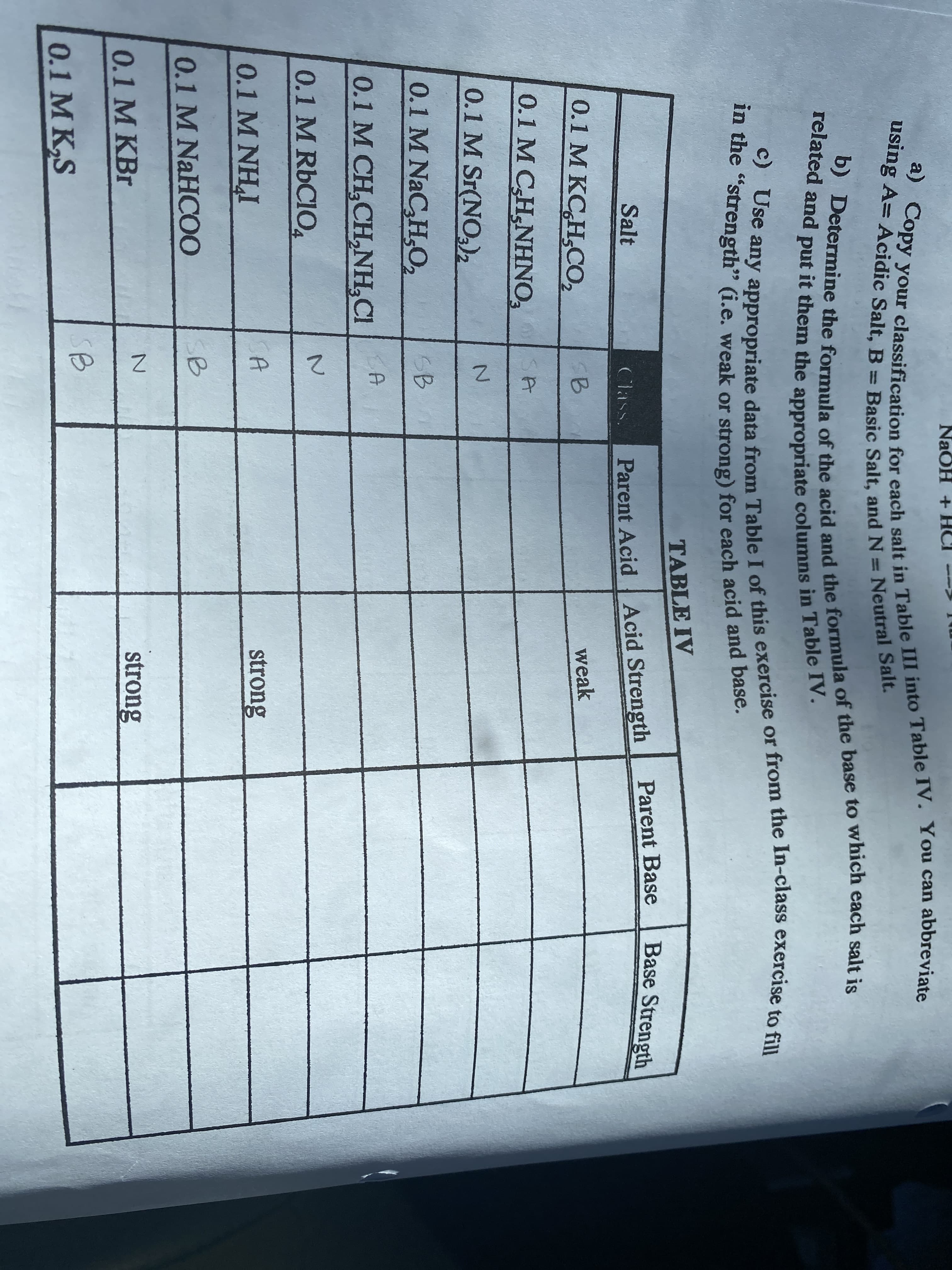
Transcribed Image Text:b) Determine the formula of the acid and the formula of the base to which each salt is
related and put it them the appropriate columns in Table IV.
using A= Acidic Salt, B = Basic Salt, and N = Neutral Salt.
a) Copy your classification for each salt in Table III into Table IV. You can abbreviate
in the "strength" (i.e. weak or strong) for each acid and base.
NaOH +
TABLE IV
Parent Base
Base Strength
Salt
Parent Acid Acid Strength
Class.
0.1 M KC,H,CO,
weak
0.1 M CH,NHNO,
0.1 M Sr(NO,)2
2.
0.1 M NaC,H,02
SB
KA
0.1 M CH,CH,NH,CI
2.
0.1 M RBCIO
4
0.1 M NHI
strong
SB
0.1 M NaHCOO
0.1 M KBr
strong
SB
0.1 M K,S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax