54.Primary standard used in the standardization of sulfuric acid a. Benzoic acid C. Sodium carbonate d. Sulfamic acid b. Potassium hydrogen phthalate
54.Primary standard used in the standardization of sulfuric acid a. Benzoic acid C. Sodium carbonate d. Sulfamic acid b. Potassium hydrogen phthalate
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.102QE
Related questions
Question
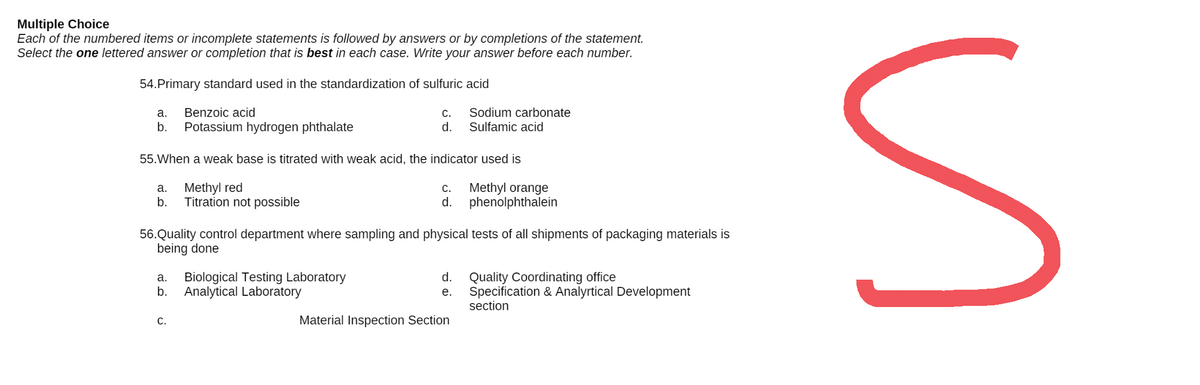
Transcribed Image Text:Multiple Choice
Each of the numbered items or incomplete statements is followed by answers or by completions of the statement.
Select the one lettered answer or completion that is best in each case. Write your answer before each number.
54.Primary standard used in the standardization of sulfuric acid
a.
Benzoic acid
C.
Sodium carbonate
Sulfamic acid
b. Potassium hydrogen phthalate
d.
55. When a weak base is titrated with weak acid, the indicator used is
a.
Methyl red
C.
Methyl orange
phenolphthalein
b.
Titration not possible
d.
56. Quality control department where sampling and physical tests of all shipments of packaging materials is
being done
a. Biological Testing Laboratory
d.
Quality Coordinating office
b. Analytical Laboratory
e.
Specification & Analyrtical Development
section
C.
Material Inspection Section
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning