6. Complex Polymer Growth a. Propose a synthesis of the following compound using a commercial carboxylic acid functionalized azo-initiator, ACVA (structure shown below), and any other reagents you may need: NC- CN HOOC, CN COOH NC NC N=N -CN b. Using the above initiator to polymerize methyl methacrylate you find that termination occurs 0.02% as frequently as monomer addition. What would you expect Mn to be (Mo = 100.1 g/mol)?
6. Complex Polymer Growth a. Propose a synthesis of the following compound using a commercial carboxylic acid functionalized azo-initiator, ACVA (structure shown below), and any other reagents you may need: NC- CN HOOC, CN COOH NC NC N=N -CN b. Using the above initiator to polymerize methyl methacrylate you find that termination occurs 0.02% as frequently as monomer addition. What would you expect Mn to be (Mo = 100.1 g/mol)?
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter24: Catalytic Carbon-carbon Bond Formation
Section: Chapter Questions
Problem 24.36P
Related questions
Question
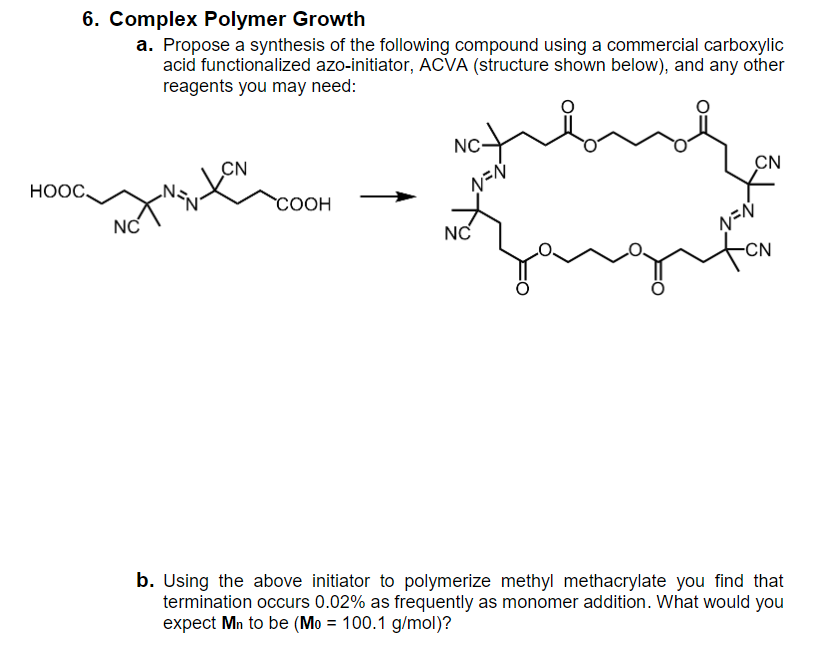
Transcribed Image Text:6. Complex Polymer Growth
a. Propose a synthesis of the following compound using a commercial carboxylic
acid functionalized azo-initiator, ACVA (structure shown below), and any other
reagents you may need:
NC-
CN
HOOC,
CN
N=N
COOH
NC
NC
N=N
-CN
b. Using the above initiator to polymerize methyl methacrylate you find that
termination occurs 0.02% as frequently as monomer addition. What would you
expect Mn to be (Mo = 100.1 g/mol)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning