(7) Once you put the spotted TLC plate into the solvent, you will see the solvent begin to move up the plate. How do you determine when the process is complete and the TLC plate should be taken out of the solvent?
(7) Once you put the spotted TLC plate into the solvent, you will see the solvent begin to move up the plate. How do you determine when the process is complete and the TLC plate should be taken out of the solvent?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section: Chapter Questions
Problem 119QRT
Related questions
Question
chart is for 8
compounds are Ethyl acetate, hexanes, silica gel, azobenzene, 4,6,8-trimethylazulene, disperse blue 14, quinazarin green and oil red O.
Transcribed Image Text:MP
°C
BP
°C
Dipole
Moment
Dielectric
Constant
Common solvents
(Debyes)
1.8
3.9
3.5
3.8
(25 °C)
78.5
48.9
37.5
36.7
35.0
32.6
24.3
100
0.0
19
water
dimethylsulfoxide "DMSO"
acetonitrile
dimethylformamide "DMF"
1,3-propanediol
methanol
ethanol
189
82
-44
-61
153
214
65
78
-27
2.5
2.87
-98
-117
1.7
20.7
20.1
17.1
56
2.7
-95
-127
acetone
17
1.7
1.5
1.7
97
118
1-propanol
1-butanol
dichloromethane
tetrahydrofuran "THF"
acetic acid
ethyl acetate
chloroform
diethyl ether
benzene
carbon tetrachloride
n-hexane
90
-97
40
8.9
65
7.4
-65
17
6.2
6.0
119
1-1.5
-84
77
1.85
-64
61
1.1
4.7
4.2
2.3
2.2
1.9
-116
35
1.25
0.0
0.0
80
-23
77
69
-95
0.0
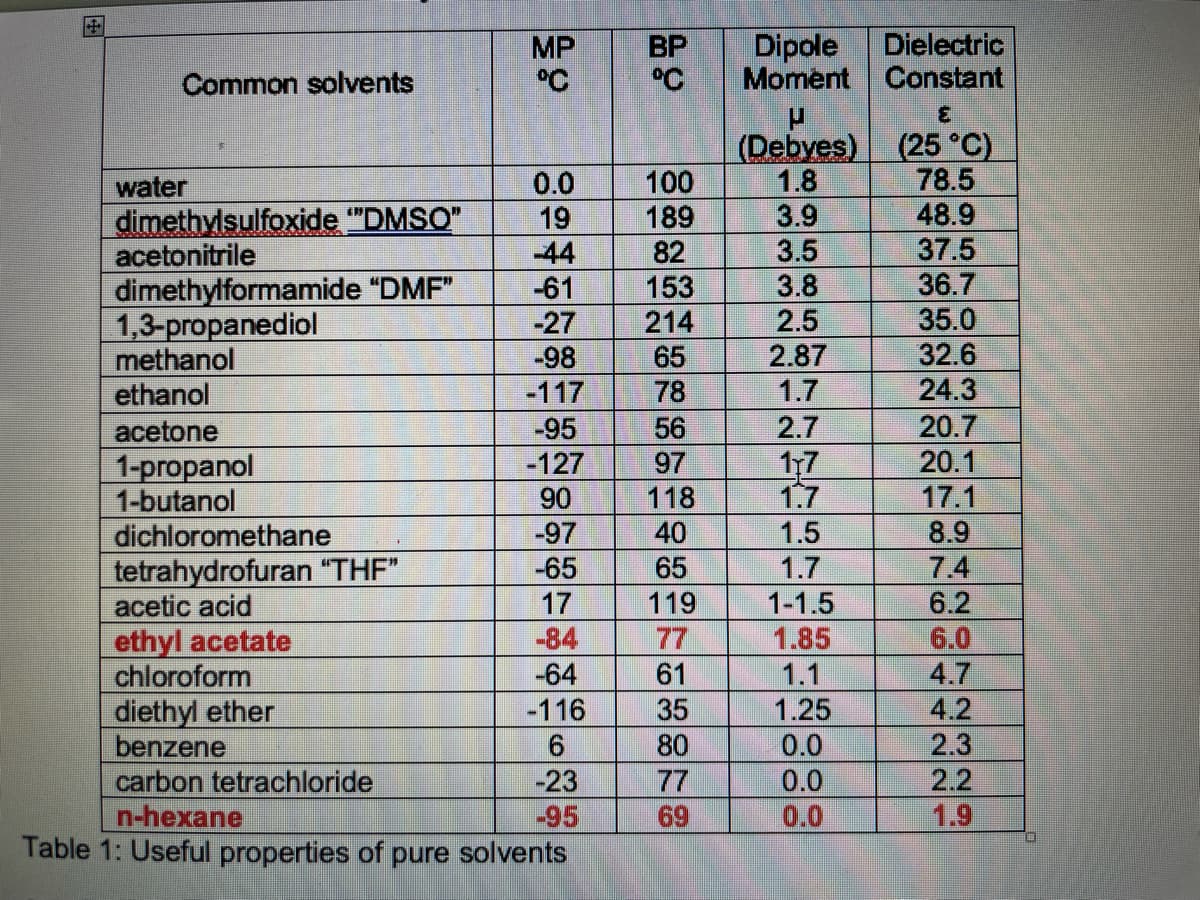
Table 1: Useful properties of pure solvents
2329
6644221
6585

Transcribed Image Text:Styles Styles
Pane
U v ab x,
Dictate
Sensitivity
(7) Once you put the spotted TLC plate into the solvent, you will see the solvent begin to move up
the plate. How do you determine when the process is complete and the TLC plate should be
taken out of the solvent?
(8) Once you take your TLC plate out of the solvent, why should you draw the line to indicate the
solvent front quickly?
(9) Just looking at the number of polarfunctional groups in the five dye molecules used in this
experiment, make an attempt to rank them (by name) from least polar to most polar here:
Lowest polarity
highest polarity
13
500 worde
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole