Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter20: Molecular Mass Spectrometry
Section: Chapter Questions
Problem 20.11QAP
Related questions
Question

Transcribed Image Text:7. What will be the appearance of a TLC plate if a solvent of too low polarity is used for the development? A solvent of
too high polarity
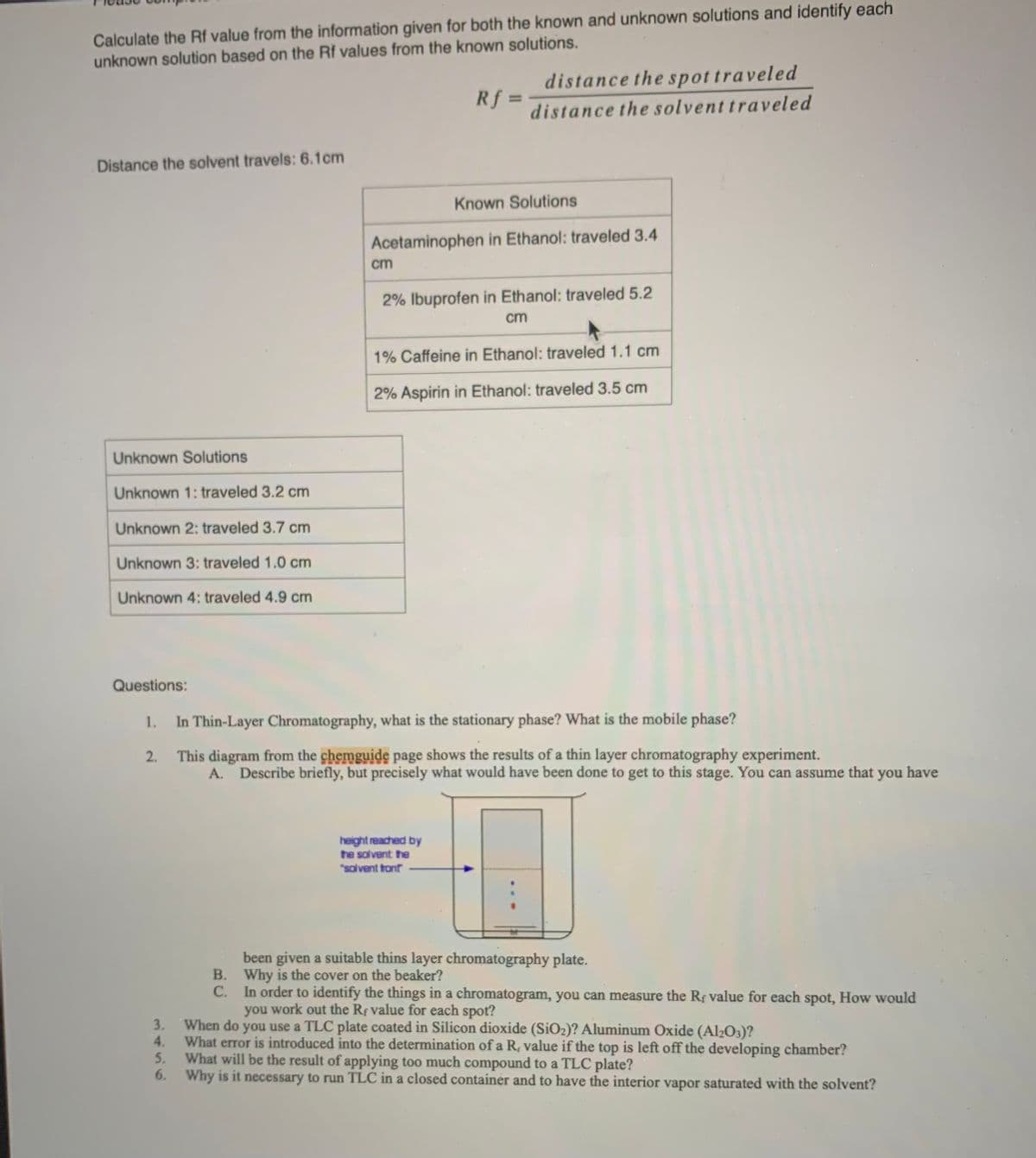
Transcribed Image Text:Calculate the Rf value from the information given for both the known and unknown solutions and identify each
unknown solution based on the Rf values from the known solutions.
distance the spottraveled
Rf =
%3D
distance the solvent traveled
Distance the solvent travels: 6.1cm
Known Solutions
Acetaminophen in Ethanol: traveled 3.4
cm
2% Ibuprofen in Ethanol: traveled 5.2
cm
1% Caffeine in Ethanol: traveled 1.1 cm
2% Aspirin in Ethanol: traveled 3.5 cm
Unknown Solutions
Unknown 1: traveled 3.2 cm
Unknown 2: traveled 3.7 cm
Unknown 3: traveled 1.0 cm
Unknown 4: traveled 4.9 cm
Questions:
In Thin-Layer Chromatography, what is the stationary phase? What is the mobile phase?
2. This diagram from the chemguide page shows the results of a thin layer chromatography experiment.
A. Describe briefly, but precisely what would have been done to get to this stage. You can assume that you have
height reached by
he solvent the
"salvent ton
been given a suitable thins layer chromatography plate.
В.
Why is the cover on the beaker?
In order to identify the things in a chromatogram, you can measure the Rf value for each spot, How would
you work out the Rf value for each spot?
С.
When do you use a TLC plate coated in Silicon dioxide (SiO2)? Aluminum Oxide (Al2O3)?
What error is introduced into the determination of a R, value if the top is left off the developing chamber?
What will be the result of applying too much compound to a TLC plate?
6. Why is it necessary to run TLC in a closed container and to have the interior vapor saturated with the solvent?
3.
1456
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning