7. Consider a cubic array of bromide ions in which the anions are touching along the face of the cube. What is the maximum radius (in angstroms) that a cation could have and still fit in the octahedral holes on the edges of the FCC unit cell?
7. Consider a cubic array of bromide ions in which the anions are touching along the face of the cube. What is the maximum radius (in angstroms) that a cation could have and still fit in the octahedral holes on the edges of the FCC unit cell?
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 5RQ: What is a lattice? What is a unit cell? Describe a simple cubic unit cell. How many net atoms are...
Related questions
Question

Transcribed Image Text:7. Consider a cubic array of bromide ions in which the anions are touching along the face of the cube. What is
the maximum radius (in angstroms) that a cation could have and still fit in the octahedral holes on the edges
of the FCC unit cell?
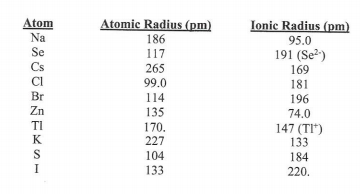
Transcribed Image Text:Atom
Atomic Radius (pm)
Ionic Radius (pm)
Na
186
95.0
Se
117
191 (Se?)
Cs
265
169
181
196
99.0
Br
114
Zn
135
170.
227
74.0
TI
147 (TI*)
133
184
K
S
104
I
133
220.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole