Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter10: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 10.41E: Verify that the following wavefunctions are indeed eigenfunctions of the Schrdinger equation, and...
Related questions
Question
100%
Chemistry.
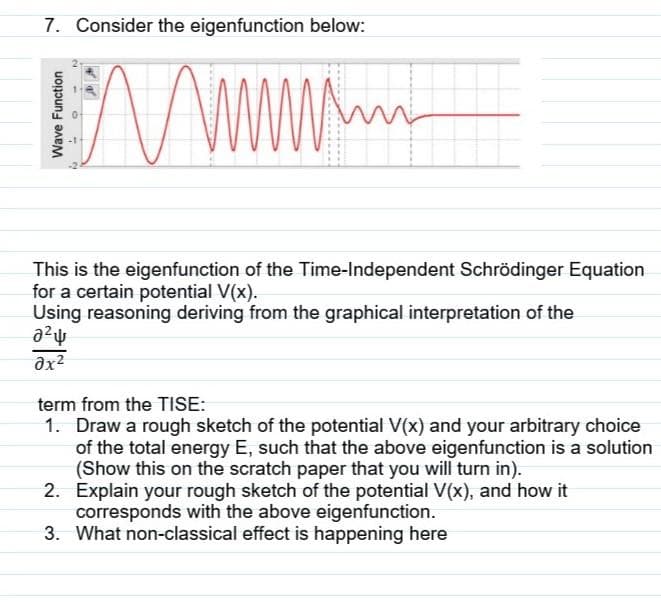
Transcribed Image Text:7. Consider the eigenfunction below:
This is the eigenfunction of the Time-Independent Schrödinger Equation
for a certain potential V(x).
Using reasoning deriving from the graphical interpretation of the
term from the TISE:
1. Draw a rough sketch of the potential V(x) and your arbitrary choice
of the total energy E, such that the above eigenfunction is a solution
(Show this on the scratch paper that you will turn in).
2. Explain your rough sketch of the potential V(x), and how it
corresponds with the above eigenfunction.
3. What non-classical effect is happening here
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning