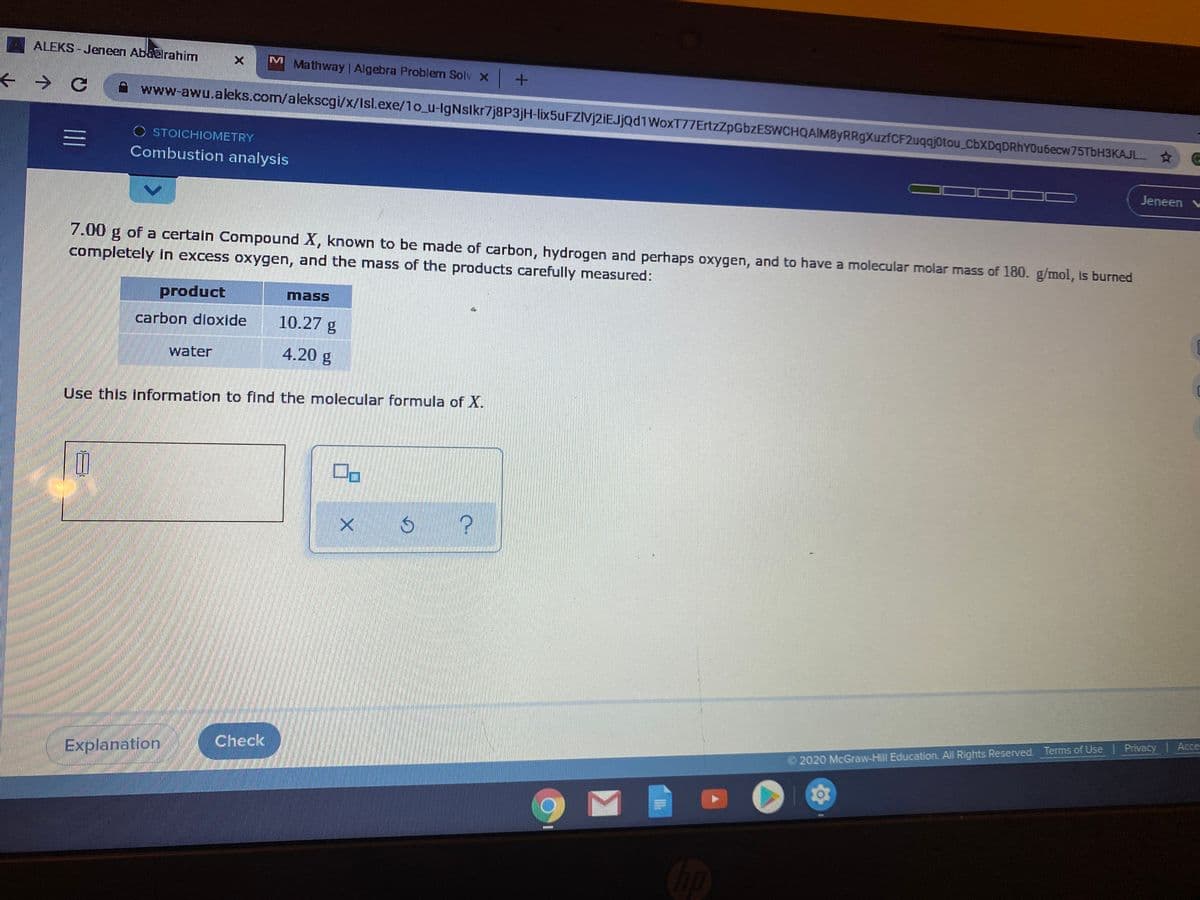
7.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 180. g/mol, Is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 10.27 g water 4.20 g Use this information to find the molecular formula of X.
7.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 180. g/mol, Is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 10.27 g water 4.20 g Use this information to find the molecular formula of X.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.30QAP
Related questions
Question
Transcribed Image Text:ALEKS-Jeneen Abaelrahim
M Mathway | Algebra Problem Solv x +
www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lix5uFZIVj2iEJjąd1WoxT77ErtzZpGbzESWCHQAIM8yRRgXuzfCF2uqqjotou_CbXDqDRhYOu6ecw75TbH3KAJL
C STOICHIOMETRY
Combustion analysis
Jeneen
7.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 180. g/mol, Is burned
completely in excess oxygen, and the mass of the products carefully measured:
product
mass
carbon dioxide
10.27 g
water
4.20 g
Use this information to find the molecular formula of X.
Check
2020 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Acce
Explanation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,