70 Nitrogen dioxide (NO2 gas and liquid water (H20) react to form aqueous nitric acid (HNO3) and nitrogen monoxide (NO) gas. Suppose you have 11.0 mo. of NO, and 13.0 mol of H20 in a reactor. 2 Suppose as much as possible of the NO, reacts. How much will be left? Round your answer to the nearest 0.1 mol. Imol x10 X Submit Assignment Continue woe ae Mook Po 04 pO DOD FA F12 esc F3 2 3 5 7 0 de T Y U P
70 Nitrogen dioxide (NO2 gas and liquid water (H20) react to form aqueous nitric acid (HNO3) and nitrogen monoxide (NO) gas. Suppose you have 11.0 mo. of NO, and 13.0 mol of H20 in a reactor. 2 Suppose as much as possible of the NO, reacts. How much will be left? Round your answer to the nearest 0.1 mol. Imol x10 X Submit Assignment Continue woe ae Mook Po 04 pO DOD FA F12 esc F3 2 3 5 7 0 de T Y U P
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 66QAP: Chlorine and fluorine react to form gaseous chlorine trifluoride. Initially, 1.75 mol of chlorine...
Related questions
Question
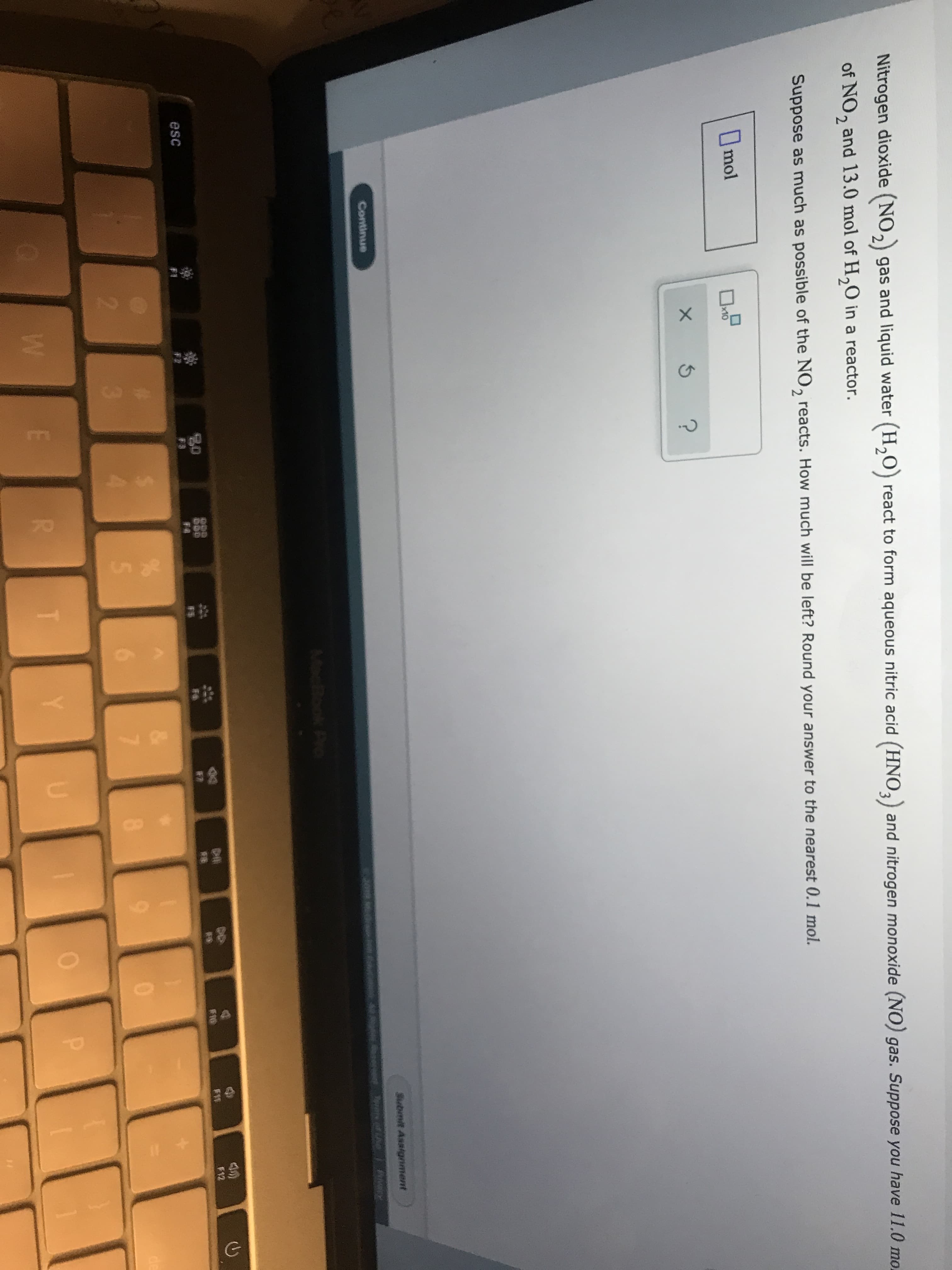
Transcribed Image Text:70
Nitrogen dioxide (NO2 gas and liquid water (H20) react to form aqueous nitric acid (HNO3) and nitrogen monoxide (NO) gas. Suppose you have 11.0 mo.
of NO, and 13.0 mol of H20 in a reactor.
2
Suppose as much as possible of the NO, reacts. How much will be left? Round your answer to the nearest 0.1 mol.
Imol
x10
X
Submit Assignment
Continue
woe ae
Mook Po
04
pO
DOD
FA
F12
esc
F3
2
3
5
7
0
de
T
Y
U
P
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning